The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster
- PMID: 28373600
- PMCID: PMC5425120
- DOI: 10.18632/aging.101218
The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster
Abstract
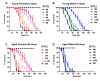
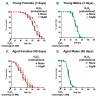
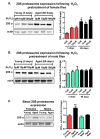
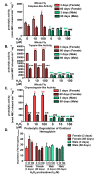
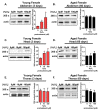
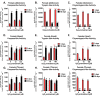
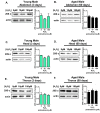
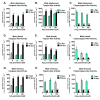
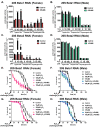
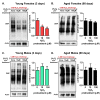
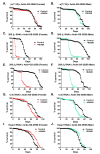
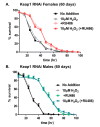
Hallmarks of aging include loss of protein homeostasis and dysregulation of stress-adaptive pathways. Loss of adaptive homeostasis, increases accumulation of DNA, protein, and lipid damage. During acute stress, the Cnc-C (Drosophila Nrf2 orthologue) transcriptionally-regulated 20S proteasome degrades damaged proteins in an ATP-independent manner. Exposure to very low, non-toxic, signaling concentrations of the redox-signaling agent hydrogen peroxide (H2O2) cause adaptive increases in the de novo expression and proteolytic activity/capacity of the 20S proteasome in female D. melanogaster (fruit-flies). Female 20S proteasome induction was accompanied by increased tolerance to a subsequent normally toxic but sub-lethal amount of H2O2, and blocking adaptive increases in proteasome expression also prevented full adaptation. We find, however, that this adaptive response is both sex- and age-dependent. Both increased proteasome expression and activity, and increased oxidative-stress resistance, in female flies, were lost with age. In contrast, male flies exhibited no H2O2 adaptation, irrespective of age. Furthermore, aging caused a generalized increase in basal 20S proteasome expression, but proteolytic activity and adaptation were both compromised. Finally, continual knockdown of Keep1 (the cytosolic inhibitor of Cnc-C) in adults resulted in older flies with greater stress resistance than their age-matched controls, but who still exhibited an age-associated loss of adaptive homeostasis.
Keywords: 20S proteasome; Nrf2; adaptive homeostasis; oxidative stress; protein aggregation; protein oxidation.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures
Similar articles
-
A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster.J Exp Biol. 2013 Feb 15;216(Pt 4):543-53. doi: 10.1242/jeb.074757. Epub 2012 Oct 4. J Exp Biol. 2013. PMID: 23038734 Free PMC article.
-
Mutations in genes cnc or dKeap1 modulate stress resistance and metabolic processes in Drosophila melanogaster.Comp Biochem Physiol A Mol Integr Physiol. 2020 Oct;248:110746. doi: 10.1016/j.cbpa.2020.110746. Epub 2020 Jun 21. Comp Biochem Physiol A Mol Integr Physiol. 2020. PMID: 32579905
-
The proteasome beta 5 subunit is essential for sexually divergent adaptive homeostatic responses to oxidative stress in D. melanogaster.Free Radic Biol Med. 2020 Nov 20;160:67-77. doi: 10.1016/j.freeradbiomed.2020.07.003. Epub 2020 Aug 3. Free Radic Biol Med. 2020. PMID: 32758664 Free PMC article.
-
Degradation of oxidized proteins by the 20S proteasome.Biochimie. 2001 Mar-Apr;83(3-4):301-10. doi: 10.1016/s0300-9084(01)01250-0. Biochimie. 2001. PMID: 11295490 Review.
-
Degradation of oxidized proteins by the proteasome: Distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways.Mol Aspects Med. 2016 Aug;50:41-55. doi: 10.1016/j.mam.2016.05.001. Epub 2016 May 4. Mol Aspects Med. 2016. PMID: 27155164 Free PMC article. Review.
Cited by
-
Tissue-Specific Knockdown of Genes of the Argonaute Family Modulates Lifespan and Radioresistance in Drosophila Melanogaster.Int J Mol Sci. 2021 Feb 27;22(5):2396. doi: 10.3390/ijms22052396. Int J Mol Sci. 2021. PMID: 33673647 Free PMC article.
-
Effects of Hyperoxia on Aging Biomarkers: A Systematic Review.Front Aging. 2022 Jan 3;2:783144. doi: 10.3389/fragi.2021.783144. eCollection 2021. Front Aging. 2022. PMID: 35822043 Free PMC article. Review.
-
Non-Genomic Hallmarks of Aging-The Review.Int J Mol Sci. 2023 Oct 23;24(20):15468. doi: 10.3390/ijms242015468. Int J Mol Sci. 2023. PMID: 37895144 Free PMC article. Review.
-
Limitations to adaptive homeostasis in an hyperoxia-induced model of accelerated ageing.Redox Biol. 2019 Jun;24:101194. doi: 10.1016/j.redox.2019.101194. Epub 2019 Apr 14. Redox Biol. 2019. PMID: 31022673 Free PMC article.
-
Deletion of Nrf2 shortens lifespan in C57BL6/J male mice but does not alter the health and survival benefits of caloric restriction.Free Radic Biol Med. 2020 May 20;152:650-658. doi: 10.1016/j.freeradbiomed.2020.01.005. Epub 2020 Jan 15. Free Radic Biol Med. 2020. PMID: 31953150 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

