Selective rescue of heightened anxiety but not gait ataxia in a premutation 90CGG mouse model of Fragile X-associated tremor/ataxia syndrome
- PMID: 28369393
- PMCID: PMC6075076
- DOI: 10.1093/hmg/ddx108
Selective rescue of heightened anxiety but not gait ataxia in a premutation 90CGG mouse model of Fragile X-associated tremor/ataxia syndrome
Abstract
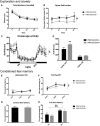
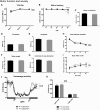
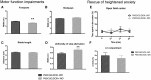
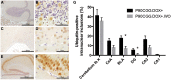
A CGG-repeat expansion in the premutation range in the Fragile X mental retardation 1 gene (FMR1) has been identified as the genetic cause of Fragile X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurodegenerative disorder that manifests with action tremor, gait ataxia and cognitive impairments. In this study, we used a bigenic mouse model, in which expression of a 90CGG premutation tract is activated in neural cells upon doxycycline administration-P90CGG mouse model. We, here, demonstrate the behavioural manifestation of clinically relevant features of FXTAS patients and premutation carrier individuals in this inducible mouse model. P90CGG mice display heightened anxiety, deficits in motor coordination and impaired gait and represent the first FXTAS model that exhibits an ataxia phenotype as observed in patients. The behavioural phenotype is accompanied by the formation of ubiquitin/FMRpolyglycine-positive intranuclear inclusions, as another hallmark of FXTAS, in the cerebellum, hippocampus and amygdala. Strikingly, upon cessation of transgene induction the anxiety phenotype of mice recovers along with a reduction of intranuclear inclusions in dentate gyrus and amygdala. In contrast, motor function deteriorates further and no reduction in intranuclear inclusions can be observed in the cerebellum. Our data thus demonstrate that expression of a 90CGG premutation expansion outside of the FMR1 context is sufficient to evoke an FXTAS-like behavioural phenotype. Brain region-specific neuropathology and (partial) behavioural reversibility make the inducible P90CGG a valuable mouse model for testing pathogenic mechanisms and therapeutic intervention methods.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Astroglial-targeted expression of the fragile X CGG repeat premutation in mice yields RAN translation, motor deficits and possible evidence for cell-to-cell propagation of FXTAS pathology.Acta Neuropathol Commun. 2019 Feb 26;7(1):27. doi: 10.1186/s40478-019-0677-7. Acta Neuropathol Commun. 2019. PMID: 30808398 Free PMC article.
-
Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency.Hum Reprod. 2016 Jan;31(1):158-68. doi: 10.1093/humrep/dev280. Epub 2015 Nov 3. Hum Reprod. 2016. PMID: 26537920 Free PMC article.
-
FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS).RNA Biol. 2004 Jul;1(2):103-5. doi: 10.4161/rna.1.2.1035. Epub 2004 Jul 17. RNA Biol. 2004. PMID: 17179750
-
What has been learned from mouse models of the Fragile X Premutation and Fragile X-associated tremor/ataxia syndrome?Clin Neuropsychol. 2016 Aug;30(6):960-72. doi: 10.1080/13854046.2016.1158254. Epub 2016 Jun 29. Clin Neuropsychol. 2016. PMID: 27355912 Free PMC article. Review.
-
Mouse models of fragile X-associated tremor ataxia.J Investig Med. 2009 Dec;57(8):837-41. doi: 10.2310/JIM.0b013e3181af59d6. J Investig Med. 2009. PMID: 19574928 Free PMC article. Review.
Cited by
-
Repeat-Associated Non-ATG Translation: Molecular Mechanisms and Contribution to Neurological Disease.Annu Rev Neurosci. 2019 Jul 8;42:227-247. doi: 10.1146/annurev-neuro-070918-050405. Epub 2019 Mar 25. Annu Rev Neurosci. 2019. PMID: 30909783 Free PMC article. Review.
-
Short antisense oligonucleotides alleviate the pleiotropic toxicity of RNA harboring expanded CGG repeats.Nat Commun. 2021 Feb 24;12(1):1265. doi: 10.1038/s41467-021-21021-w. Nat Commun. 2021. PMID: 33627639 Free PMC article.
-
Mouse models of fragile X-related disorders.Dis Model Mech. 2023 Feb 1;16(2):dmm049485. doi: 10.1242/dmm.049485. Epub 2023 Jan 24. Dis Model Mech. 2023. PMID: 36692473 Free PMC article. Review.
-
Neurodegenerative diseases associated with non-coding CGG tandem repeat expansions.Nat Rev Neurol. 2022 Mar;18(3):145-157. doi: 10.1038/s41582-021-00612-7. Epub 2022 Jan 12. Nat Rev Neurol. 2022. PMID: 35022573 Review.
-
In silico, in vitro, and in vivo Approaches to Identify Molecular Players in Fragile X Tremor and Ataxia Syndrome.Front Mol Biosci. 2020 Mar 11;7:31. doi: 10.3389/fmolb.2020.00031. eCollection 2020. Front Mol Biosci. 2020. PMID: 32219099 Free PMC article. Review.
References
-
- Hagerman R.J., Leehey M., Heinrichs W., Tassone F., Wilson R., Hills J., Grigsby J., Gage B., Hagerman P.J. (2001) Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology, 57, 127–130. - PubMed
-
- Jacquemont S., Hagerman R.J., Leehey M.A., Hall D.A., Levine R.A., Brunberg J.A., Zhang L., Jardini T., Gane L.W., Harris S.W.. et al. (2004) Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA, 291, 460–469. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

