Hidden Mode of Action of Glycopeptide Antibiotics: Inhibition of Wall Teichoic Acid Biosynthesis
- PMID: 28368603
- PMCID: PMC6013045
- DOI: 10.1021/acs.jpcb.7b00324
Hidden Mode of Action of Glycopeptide Antibiotics: Inhibition of Wall Teichoic Acid Biosynthesis
Abstract
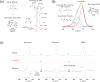
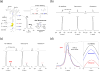
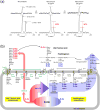
Glycopeptide antibiotics inhibit the peptidoglycan biosynthesis in Gram-positive bacteria by targeting lipid II. This prevents the recycling of bactoprenol phosphate, the lipid transporter that is shared by peptidoglycan and wall teichoic acid biosyntheses. In this study, we investigate the effects of glycopeptide antibiotics on peptidoglycan and wall teichoic acid biosynthesis. The incorporation of d-[1-13C]alanine, d-[15N]alanine, and l-[1-13C]lysine into peptidoglycan and wall teichoic acid in intact whole cells of Staphylococcus aureus was measured using 13C{15N} and 15N{13C} rotational-echo double resonance NMR. S. aureus treated with oritavancin and vancomycin at subminimal inhibitory concentrations exhibit a large reduction in d-Ala incorporation into wall teichoic acid, but without changes to the peptidoglycan cross-links or the stem-links. Thus, sequestration of bactoprenol phosphate by glycopeptide antibiotics resulted in inhibition of d-Ala incorporation into the wall teichoic acid prior to the inhibition of peptidoglycan biosynthesis. Our finding shows that S. aureus responds to glycopeptide-induced cell wall stress by routing all available d-Ala to the peptidoglycan biosynthesis, at the cost of reducing the wall teichoic acid biosynthesis.
Figures

Similar articles
-
Inhibition of d-Ala incorporation into wall teichoic acid in Staphylococcus aureus by desleucyl-oritavancin.Chem Commun (Camb). 2017 May 18;53(41):5649-5652. doi: 10.1039/c7cc02635h. Chem Commun (Camb). 2017. PMID: 28480909 Free PMC article.
-
Solid-state NMR characterization of amphomycin effects on peptidoglycan and wall teichoic acid biosyntheses in Staphylococcus aureus.Sci Rep. 2016 Aug 19;6:31757. doi: 10.1038/srep31757. Sci Rep. 2016. PMID: 27538449 Free PMC article.
-
Inhibition of Staphylococcus aureus Cell Wall Biosynthesis by Desleucyl-Oritavancin: a Quantitative Peptidoglycan Composition Analysis by Mass Spectrometry.J Bacteriol. 2017 Jul 11;199(15):e00278-17. doi: 10.1128/JB.00278-17. Print 2017 Aug 1. J Bacteriol. 2017. PMID: 28507244 Free PMC article.
-
Teichoic acid biosynthesis as an antibiotic target.Curr Opin Microbiol. 2013 Oct;16(5):531-7. doi: 10.1016/j.mib.2013.06.014. Epub 2013 Jul 31. Curr Opin Microbiol. 2013. PMID: 23916223 Free PMC article. Review.
-
Inhibitors targeting on cell wall biosynthesis pathway of MRSA.Mol Biosyst. 2012 Nov;8(11):2828-38. doi: 10.1039/c2mb25188d. Epub 2012 Aug 16. Mol Biosyst. 2012. PMID: 22898792 Review.
Cited by
-
Multitarget Approaches against Multiresistant Superbugs.ACS Infect Dis. 2020 Jun 12;6(6):1346-1365. doi: 10.1021/acsinfecdis.0c00001. Epub 2020 Mar 19. ACS Infect Dis. 2020. PMID: 32156116 Free PMC article. Review.
-
Antibiotic-induced accumulation of lipid II synergizes with antimicrobial fatty acids to eradicate bacterial populations.Elife. 2023 Mar 6;12:e80246. doi: 10.7554/eLife.80246. Elife. 2023. PMID: 36876902 Free PMC article.
-
Molecular dynamics simulations of the secondary-binding site in disaccharide-modified glycopeptide antibiotics.Sci Rep. 2022 Apr 30;12(1):7087. doi: 10.1038/s41598-022-10735-6. Sci Rep. 2022. PMID: 35490171 Free PMC article.
-
Breaking down the cell wall: Still an attractive antibacterial strategy.Front Microbiol. 2022 Sep 23;13:952633. doi: 10.3389/fmicb.2022.952633. eCollection 2022. Front Microbiol. 2022. PMID: 36212892 Free PMC article. Review.
-
Synthetic and Semisynthetic Compounds as Antibacterials Targeting Virulence Traits in Resistant Strains: A Narrative Updated Review.Antibiotics (Basel). 2023 May 25;12(6):963. doi: 10.3390/antibiotics12060963. Antibiotics (Basel). 2023. PMID: 37370282 Free PMC article. Review.
References
-
- Brotz H, Bierbaum G, Reynolds PE, Sahl HG. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur J Biochem. 1997;246:193–199. - PubMed
-
- Cegelski L, Kim SJ, Hing AW, Studelska DR, O’Connor RD, Mehta AK, Schaefer J. Rotational-echo double resonance characterization of the effects of vancomycin on cell wall synthesis in Staphylococcus aureus. Biochemistry. 2002;41:13053–13058. - PubMed
-
- Cooper DG, Snyder NJ, Zweifel MJ, Staszak MA, Wilkie SC, Nicas TI, Mullen DL, Butler TF, Rodriguez MJ, Huff BE, Thompson CR. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J Antibiot. 1996;49:575–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

