Boosting of Cross-Reactive and Protection-Associated T Cells in Children After Live Attenuated Influenza Vaccination
- PMID: 28368530
- PMCID: PMC5461427
- DOI: 10.1093/infdis/jix165
Boosting of Cross-Reactive and Protection-Associated T Cells in Children After Live Attenuated Influenza Vaccination
Abstract
Background: Live attenuated influenza vaccines (LAIVs) stimulate a multifaceted immune response including cellular immunity, which may provide protection against newly emerging strains. This study shows proof of concept that LAIVs boost preexisting, cross-reactive T cells in children to genetically diverse influenza A virus (IAV) strains to which the children had not been exposed.
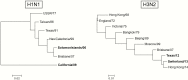
Methods: We studied the long-term cross-reactive T-cell response in 14 trivalent LAIV-vaccinated children using the fluorescent immunospot assay (FluoroSpot) with heterologous H1N1 and H3N2 IAVs and CD8+ peptides from the internal proteins (matrix protein 1 [M1], nucleoprotein [NP], polymerase basic protein 1 [PB1]). Serum antibody responses were determined by means of hemagglutination inhibition assay. Blood samples were collected before vaccination and up to 1 year after vaccination.
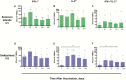
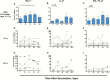
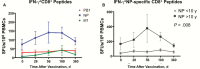
Results: Preexisting cross-reactive T cells to genetically diverse IAV strains were found in the majority of the children, which were further boosted in 50% of them after receipt of LAIV. Further analyses of these T cells showed significant increases in CD8+ T cells, mainly dominated by NP-specific responses. After vaccination with LAIV, the youngest children showed the highest increase in T-cell responses.
Conclusion: LAIV boosts durable, cross-reactive T-cell responses in children and may have a clinically protective effect at the population level. LAIV may be a first step toward the desired universal influenza vaccine.
Keywords: Influenza; LAIV; T-cell; cellular immune response; children; cross-reactive; heterologous; protection.; vaccine.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Similar articles
-
Rationale for vaccination with trivalent or quadrivalent live attenuated influenza vaccines: Protective vaccine efficacy in the ferret model.PLoS One. 2018 Dec 3;13(12):e0208028. doi: 10.1371/journal.pone.0208028. eCollection 2018. PLoS One. 2018. PMID: 30507951 Free PMC article.
-
Oral Fluids as a Live-Animal Sample Source for Evaluating Cross-Reactivity and Cross-Protection following Intranasal Influenza A Virus Vaccination in Pigs.Clin Vaccine Immunol. 2015 Oct;22(10):1109-20. doi: 10.1128/CVI.00358-15. Epub 2015 Aug 19. Clin Vaccine Immunol. 2015. PMID: 26291090 Free PMC article.
-
Early Induction of Cross-Reactive CD8+ T-Cell Responses in Tonsils After Live-Attenuated Influenza Vaccination in Children.J Infect Dis. 2020 Apr 7;221(9):1528-1537. doi: 10.1093/infdis/jiz583. J Infect Dis. 2020. PMID: 32255493 Free PMC article.
-
The Quest for a Truly Universal Influenza Vaccine.Front Cell Infect Microbiol. 2019 Oct 10;9:344. doi: 10.3389/fcimb.2019.00344. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31649895 Free PMC article. Review.
-
Novel Approaches for The Development of Live Attenuated Influenza Vaccines.Viruses. 2019 Feb 22;11(2):190. doi: 10.3390/v11020190. Viruses. 2019. PMID: 30813325 Free PMC article. Review.
Cited by
-
Systemic prime mucosal boost significantly increases protective efficacy of bivalent RSV influenza viral vectored vaccine.NPJ Vaccines. 2024 Jun 26;9(1):118. doi: 10.1038/s41541-024-00912-1. NPJ Vaccines. 2024. PMID: 38926455 Free PMC article.
-
Immune Responses Elicited by Live Attenuated Influenza Vaccines as Correlates of Universal Protection against Influenza Viruses.Vaccines (Basel). 2021 Apr 7;9(4):353. doi: 10.3390/vaccines9040353. Vaccines (Basel). 2021. PMID: 33916924 Free PMC article. Review.
-
Respiratory virus-induced heterologous immunity: Part of the problem or part of the solution?Allergo J. 2018;27(3):28-45. doi: 10.1007/s15007-018-1580-4. Epub 2018 Apr 26. Allergo J. 2018. PMID: 32300267 Free PMC article. Review.
-
Intranasal Live Influenza Vaccine Priming Elicits Localized B Cell Responses in Mediastinal Lymph Nodes.J Virol. 2018 Apr 13;92(9):e01970-17. doi: 10.1128/JVI.01970-17. Print 2018 May 1. J Virol. 2018. PMID: 29444938 Free PMC article.
-
Impact of Pre-Existing Immunity to Influenza on Live-Attenuated Influenza Vaccine (LAIV) Immunogenicity.Vaccines (Basel). 2020 Nov 16;8(4):683. doi: 10.3390/vaccines8040683. Vaccines (Basel). 2020. PMID: 33207559 Free PMC article.
References
-
- Wilkinson TM, Li CK, Chui CS, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274–80. - PubMed
-
- Sridhar S, Begom S, Bermingham A, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19:1305–12. - PubMed
-
- World Health Organization. Fact sheet about seasonal influenza. 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

