Crystal structure of a small heat-shock protein from Xylella fastidiosa reveals a distinct high-order structure
- PMID: 28368281
- PMCID: PMC5379172
- DOI: 10.1107/S2053230X17004101
Crystal structure of a small heat-shock protein from Xylella fastidiosa reveals a distinct high-order structure
Abstract
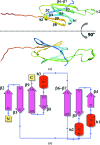
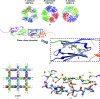
Citrus variegated chlorosis is a disease that attacks economically important citrus plantations and is caused by the plant-pathogenic bacterium Xylella fastidiosa. In this work, the structure of a small heat-shock protein from X. fastidiosa (XfsHSP17.9) is reported. The high-order structures of small heat-shock proteins from other organisms are arranged in the forms of double-disc, hollow-sphere or spherical assemblies. Unexpectedly, the structure reported here reveals a high-order architecture forming a nearly square cavity.
Keywords: XfsHSP17.9; Xylella fastidiosa; chaperones; citrus variegated chlorosis; small heat-shock protein; α-crystallin domain.
Figures
Similar articles
-
Initial crystallographic studies of a small heat-shock protein from Xylella fastidiosa.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 May 1;68(Pt 5):535-9. doi: 10.1107/S1744309112009347. Epub 2012 Apr 20. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 22691782 Free PMC article.
-
Crystallization and preliminary X-ray analysis of stationary phase survival protein E (SurE) from Xylella fastidiosa in two crystal forms.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Apr 1;68(Pt 4):464-7. doi: 10.1107/S1744309112007129. Epub 2012 Mar 28. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 22505421 Free PMC article.
-
Expression and purification of a small heat shock protein from the plant pathogen Xylella fastidiosa.Protein Expr Purif. 2004 Feb;33(2):297-303. doi: 10.1016/j.pep.2003.10.007. Protein Expr Purif. 2004. PMID: 14711518
-
Absence of classical heat shock response in the citrus pathogen Xylella fastidiosa.Curr Microbiol. 2007 Feb;54(2):119-23. doi: 10.1007/s00284-006-0215-2. Epub 2007 Jan 5. Curr Microbiol. 2007. PMID: 17211542
-
Small heat shock proteins: big folding machines.Cell Stress Chaperones. 2015 Mar;20(2):207-12. doi: 10.1007/s12192-014-0561-0. Epub 2014 Dec 24. Cell Stress Chaperones. 2015. PMID: 25536931 Free PMC article. Review.
Cited by
-
What Can De Novo Protein Design Bring to the Treatment of Hematological Disorders?Biology (Basel). 2023 Jan 20;12(2):166. doi: 10.3390/biology12020166. Biology (Basel). 2023. PMID: 36829445 Free PMC article. Review.
-
Genome-wide screens identify Toxoplasma gondii determinants of parasite fitness in IFNγ-activated murine macrophages.Nat Commun. 2020 Oct 16;11(1):5258. doi: 10.1038/s41467-020-18991-8. Nat Commun. 2020. PMID: 33067458 Free PMC article.
References
-
- Azzoni, A. R., Tada, S. F. S., Rosselli, L. K., Paula, D. P., Catani, C. F., Sabino, A. A., Barbosa, J. A. R. G., Guimarães, B. G., Eberlin, M. N., Medrano, F. J. & Souza, A. P. (2004). Protein Expr. Purif. 33, 297–303. - PubMed
-
- Bagnéris, C., Bateman, O. A., Naylor, C. E., Cronin, N., Boelens, W. C., Keep, N. H. & Slingsby, C. (2009). J. Mol. Biol. 392, 1242–1252. - PubMed
MeSH terms
Substances
Grants and funding
- 2008/52197-4/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- 2001/07533-7/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- 2002/01316-7/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- 2008/55690-3/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- 2006/52844-4/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

