Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway
- PMID: 28358365
- PMCID: PMC5386545
- DOI: 10.1038/cddis.2017.150
Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway
Abstract
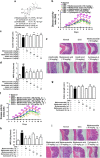
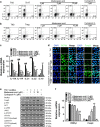
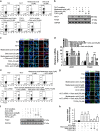
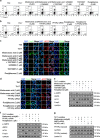
The imbalance between Th17 and Treg cells substantially contributes to the intestinal immune disturbance and subsequent tissue injury in ulcerative colitis. The triterpenoid-rich fraction of Centella asiatica was able to ameliorate dextran sulfate sodium-induced colitis in mice. Here we explored its active ingredient and underlying mechanism with a focus on restoring the Th17/Treg balance. The four main triterpenoids occurring in C. asiatica were shown to attenuate colitis in mice by oral administration. The most effective ingredient madecassoside lost anti-colitis effect when applied topically in the colon, and madecassic acid was recognized to be the active form of madecassoside. Oral administration of madecassic acid decreased the percentage of Th17 cells and downregulated the expression of RORγt, IL-17A, IL-17F, IL-21 and IL-22 and increased the percentage of Treg cells and the expression of Foxp3 and IL-10 in the colons of mice with colitis, but it did not affect Th1 and Th2 cells. Under Th17-polarizing conditions, madecassic acid downregulated ACC1 expression and enhanced the shift of Th17 cells toward Treg cells, but it did not affect the differentiation of Treg cells under Treg-polarizing conditions. Both compound C and AMPK siRNA inhibited the madecassic acid-mediated downregulation of ACC1 expression and shift of Th17 cells to Treg cells under Th17-polarizing conditions. GW9662, T0070907 and PPARγ siRNA blocked the effect of madecassic acid on AMPK activation, ACC1 expression and shift of Th17 cells to Treg cells. Furthermore, madecassic acid was identified as a PPARγ agonist, as it promoted PPARγ transactivation. The correlation between activation of PPARγ and AMPK, downregulation of ACC1 expression, restoration of Th17/Treg balance and attenuation of colitis by madecassic acid was validated in mice with DSS-induced colitis. In conclusion, madecassic acid was the active form of madecassoside in ameliorating colitis by restoring the Th17/Treg balance via regulating the PPARγ/AMPK/ACC1 pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway.Int Immunopharmacol. 2015 Dec;29(2):393-400. doi: 10.1016/j.intimp.2015.10.024. Epub 2015 Oct 26. Int Immunopharmacol. 2015. PMID: 26514300
-
Feruloylated Oligosaccharides Alleviate Dextran Sulfate Sodium-Induced Colitis in Vivo.J Agric Food Chem. 2019 Aug 28;67(34):9522-9531. doi: 10.1021/acs.jafc.9b03647. Epub 2019 Aug 16. J Agric Food Chem. 2019. PMID: 31379161
-
Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance.Biomed Pharmacother. 2019 Jan;109:2396-2408. doi: 10.1016/j.biopha.2018.11.087. Epub 2018 Nov 30. Biomed Pharmacother. 2019. PMID: 30551499
-
The Roles of T Helper 1, T Helper 17 and Regulatory T Cells in the Pathogenesis of Sarcoidosis.Iran J Allergy Asthma Immunol. 2016 Aug;15(4):334-339. Iran J Allergy Asthma Immunol. 2016. PMID: 27921415 Review.
-
Immuno-metabolic control of the balance between Th17-polarized and regulatory T-cells during HIV infection.Cytokine Growth Factor Rev. 2023 Feb;69:1-13. doi: 10.1016/j.cytogfr.2023.01.001. Epub 2023 Jan 18. Cytokine Growth Factor Rev. 2023. PMID: 36681548 Review.
Cited by
-
BMSCs improve TNBS-induced colitis in rats by inducing Treg differentiation by expressing PD-L1.Biotechnol Lett. 2022 Nov;44(11):1263-1275. doi: 10.1007/s10529-022-03307-1. Epub 2022 Oct 20. Biotechnol Lett. 2022. PMID: 36261682 Free PMC article.
-
Metabolic regulation of the immune system in health and diseases: mechanisms and interventions.Signal Transduct Target Ther. 2024 Oct 9;9(1):268. doi: 10.1038/s41392-024-01954-6. Signal Transduct Target Ther. 2024. PMID: 39379377 Free PMC article. Review.
-
Network Pharmacology-Based Validation of the Efficacy of Huiyangjiuji Decoction in the Treatment of Experimental Colitis.Front Pharmacol. 2021 May 28;12:666432. doi: 10.3389/fphar.2021.666432. eCollection 2021. Front Pharmacol. 2021. PMID: 34122086 Free PMC article.
-
A metabolomic platform to identify and quantify polyphenols in coffee and related species using liquid chromatography mass spectrometry.Front Plant Sci. 2023 Jan 6;13:1057645. doi: 10.3389/fpls.2022.1057645. eCollection 2022. Front Plant Sci. 2023. PMID: 36684722 Free PMC article.
-
The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient "Yin-Yang" theory and modern immunology.Cell Commun Signal. 2024 Feb 6;22(1):99. doi: 10.1186/s12964-024-01505-0. Cell Commun Signal. 2024. PMID: 38317142 Free PMC article. Review.
References
-
- Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Rev 2014; 13: 463–466. - PubMed
-
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015; 12: 205–217. - PubMed
-
- De Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016; 13: 13–27. - PubMed
-
- Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol 2010; 30: 80–89. - PubMed
-
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474: 298–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

