Pathogenic Events in a Nonhuman Primate Model of Oral Poliovirus Infection Leading to Paralytic Poliomyelitis
- PMID: 28356537
- PMCID: PMC5487571
- DOI: 10.1128/JVI.02310-16
Pathogenic Events in a Nonhuman Primate Model of Oral Poliovirus Infection Leading to Paralytic Poliomyelitis
Abstract
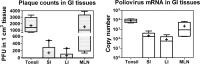
Despite a great deal of prior research, the early pathogenic events in natural oral poliovirus infection remain poorly defined. To establish a model for study, we infected 39 macaques by feeding them single high doses of the virulent Mahoney strain of wild type 1 poliovirus. Doses ranging from 107 to 109 50% tissue culture infective doses (TCID50) consistently infected all the animals, and many monkeys receiving 108 or 109 TCID50 developed paralysis. There was no apparent difference in the susceptibilities of the three macaque species (rhesus, cynomolgus, and bonnet) used. Virus excretion in stool and nasopharynges was consistently observed, with occasional viremia, and virus was isolated from tonsils, gut mucosa, and draining lymph nodes. Viral replication proteins were detected in both epithelial and lymphoid cell populations expressing CD155 in the tonsil and intestine, as well as in spinal cord neurons. Necrosis was observed in these three cell types, and viral replication in the tonsil/gut was associated with histopathologic destruction and inflammation. The sustained response of neutralizing antibody correlated temporally with resolution of viremia and termination of virus shedding in oropharynges and feces. For the first time, this model demonstrates that early in the infectious process, poliovirus replication occurs in both epithelial cells (explaining virus shedding in the gastrointestinal tract) and lymphoid/monocytic cells in tonsils and Peyer's patches (explaining viremia), extending previous studies of poliovirus pathogenesis in humans. Because the model recapitulates human poliovirus infection and poliomyelitis, it can be used to study polio pathogenesis and to assess the efficacy of candidate antiviral drugs and new vaccines.IMPORTANCE Early pathogenic events of poliovirus infection remain largely undefined, and there is a lack of animal models mimicking natural oral human infection leading to paralytic poliomyelitis. All 39 macaques fed with single high doses ranging from 107 to 109 TCID50 Mahoney type 1 virus were infected, and many of the monkeys developed paralysis. Virus excretion in stool and nasopharynges was consistently observed, with occasional viremia; tonsil, mesentery lymph nodes, and intestinal mucosa served as major target sites of viral replication. For the first time, this model demonstrates that early in the infectious process, poliovirus replication occurs in both epithelial cells (explaining virus shedding in the gastrointestinal tract) and lymphoid/monocytic cells in tonsils and Peyer's patches (explaining viremia), thereby supplementing historical reconstructions of poliovirus pathogenesis. Because the model recapitulates human poliovirus infection and poliomyelitis, it can be used to study polio pathogenesis, candidate antiviral drugs, and the efficacy of new vaccines.
Keywords: animal model; macaques; oral poliovirus infection; poliomyelitis.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Sabin attenuated LSc/2ab strain of poliovirus spreads to the spinal cord from a peripheral nerve in bonnet monkeys (Macaca radiata).J Gen Virol. 2001 Jun;82(Pt 6):1329-1338. doi: 10.1099/0022-1317-82-6-1329. J Gen Virol. 2001. PMID: 11369876
-
Experimental poliomyelitis in bonnet monkey. Clinical features, virology and pathology.Dev Biol Stand. 1993;78:71-8. Dev Biol Stand. 1993. PMID: 8388833
-
Immunofluorescence analysis of poliovirus receptor expression in Peyer's patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection.J Infect Dis. 2002 Sep 1;186(5):585-92. doi: 10.1086/342682. Epub 2002 Aug 9. J Infect Dis. 2002. PMID: 12195344
-
The pathogenesis of poliomyelitis: what we don't know.Adv Virus Res. 2008;71:1-50. doi: 10.1016/S0065-3527(08)00001-8. Adv Virus Res. 2008. PMID: 18585526 Review.
-
New insights into physiopathology of immunodeficiency-associated vaccine-derived poliovirus infection; systematic review of over 5 decades of data.Vaccine. 2018 Mar 20;36(13):1711-1719. doi: 10.1016/j.vaccine.2018.02.059. Epub 2018 Feb 23. Vaccine. 2018. PMID: 29478755 Review.
Cited by
-
Coxsackievirus B3 HFMD animal models in Syrian hamster and rhesus monkey.Virol Sin. 2024 Apr;39(2):290-300. doi: 10.1016/j.virs.2024.02.001. Epub 2024 Feb 6. Virol Sin. 2024. PMID: 38331038 Free PMC article.
-
Is it time to switch to a formulation other than the live attenuated poliovirus vaccine to prevent poliomyelitis?Front Public Health. 2024 Jan 8;11:1284337. doi: 10.3389/fpubh.2023.1284337. eCollection 2023. Front Public Health. 2024. PMID: 38259741 Free PMC article. Review.
-
Human B cells and dendritic cells are susceptible and permissive to enterovirus D68 infection.mSphere. 2024 Feb 28;9(2):e0052623. doi: 10.1128/msphere.00526-23. Epub 2024 Jan 23. mSphere. 2024. PMID: 38259063 Free PMC article.
-
Early enterovirus translation deficits extend viral RNA replication and elicit sustained MDA5-directed innate signaling.mBio. 2023 Dec 19;14(6):e0191523. doi: 10.1128/mbio.01915-23. Epub 2023 Nov 14. mBio. 2023. PMID: 37962360 Free PMC article.
-
Recombinant polio-rhinovirus immunotherapy for recurrent paediatric high-grade glioma: a phase 1b trial.Lancet Child Adolesc Health. 2023 Jul;7(7):471-478. doi: 10.1016/S2352-4642(23)00031-7. Epub 2023 Mar 30. Lancet Child Adolesc Health. 2023. PMID: 37004712 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

