Pneumocystis-Driven Inducible Bronchus-Associated Lymphoid Tissue Formation Requires Th2 and Th17 Immunity
- PMID: 28355561
- PMCID: PMC5411079
- DOI: 10.1016/j.celrep.2017.03.016
Pneumocystis-Driven Inducible Bronchus-Associated Lymphoid Tissue Formation Requires Th2 and Th17 Immunity
Abstract
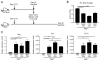
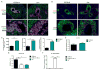
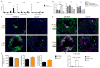
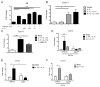
Inducible bronchus-associated lymphoid tissue (iBALT) is an ectopic lymphoid structure composed of highly organized T cell and B cell zones that forms in the lung in response to infectious or inflammatory stimuli. Here, we develop a model for fungal-mediated iBALT formation, using infection with Pneumocystis that induces development of pulmonary lymphoid follicles. Pneumocystis-dependent iBALT structure formation and organization required CXCL13 signaling. Cxcl13 expression was regulated by interleukin (IL)-17 family members, as Il17ra-/-, Il17rb-/-, and Il17rc-/- mice failed to develop iBALT. Interestingly, Il17rb-/- mice have intact Th17 responses, but failed to generate an anti-Pneumocystis Th2 response. Given a role for Th2 and Th17 immunity in iBALT formation, we demonstrated that primary pulmonary fibroblasts synergistically upregulated Cxcl13 transcription following dual stimulation with IL-13 and IL-17A in a STAT3/GATA3-dependent manner. Together, these findings uncover a role for Th2/Th17 cells in regulating Cxcl13 expression and provide an experimental model for fungal-driven iBALT formation.
Keywords: Cxcl13; Pneumocystis; Th17; Th2; fungus; iBALT; immunity; lymphoid tissue.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Inducible Bronchus-Associated Lymphoid Tissue (iBALT) Attenuates Pulmonary Pathology in a Mouse Model of Allergic Airway Disease.Front Immunol. 2020 Sep 25;11:570661. doi: 10.3389/fimmu.2020.570661. eCollection 2020. Front Immunol. 2020. PMID: 33101290 Free PMC article.
-
The development of inducible bronchus-associated lymphoid tissue depends on IL-17.Nat Immunol. 2011 Jun 12;12(7):639-46. doi: 10.1038/ni.2053. Nat Immunol. 2011. PMID: 21666689 Free PMC article.
-
Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity.Nat Med. 2004 Sep;10(9):927-34. doi: 10.1038/nm1091. Epub 2004 Aug 15. Nat Med. 2004. PMID: 15311275
-
Regulation of inducible BALT formation and contribution to immunity and pathology.Mucosal Immunol. 2010 Nov;3(6):537-44. doi: 10.1038/mi.2010.52. Epub 2010 Sep 1. Mucosal Immunol. 2010. PMID: 20811344 Review.
-
Friend or Foe: The Protective and Pathological Roles of Inducible Bronchus-Associated Lymphoid Tissue in Pulmonary Diseases.J Immunol. 2019 May 1;202(9):2519-2526. doi: 10.4049/jimmunol.1801135. J Immunol. 2019. PMID: 31010841 Free PMC article. Review.
Cited by
-
Updates on T helper type 17 immunity in respiratory disease.Immunology. 2019 Jan;156(1):3-8. doi: 10.1111/imm.13006. Epub 2018 Oct 24. Immunology. 2019. PMID: 30260473 Free PMC article. Review.
-
Application of light sheet microscopy for qualitative and quantitative analysis of bronchus-associated lymphoid tissue in mice.Cell Mol Immunol. 2018 Oct;15(10):875-887. doi: 10.1038/cmi.2017.150. Epub 2018 Feb 12. Cell Mol Immunol. 2018. PMID: 29429996 Free PMC article.
-
Vaccine-driven lung TRM cells provide immunity against Klebsiella via fibroblast IL-17R signaling.Sci Immunol. 2021 Sep 10;6(63):eabf1198. doi: 10.1126/sciimmunol.abf1198. Epub 2021 Sep 10. Sci Immunol. 2021. PMID: 34516780 Free PMC article.
-
Cryptococcus neoformans Evades Pulmonary Immunity by Modulating Xylose Precursor Transport.Infect Immun. 2020 Jul 21;88(8):e00288-20. doi: 10.1128/IAI.00288-20. Print 2020 Jul 21. Infect Immun. 2020. PMID: 32423915 Free PMC article.
-
Contribution of adaptive immunity to human COPD and experimental models of emphysema.Physiol Rev. 2023 Apr 1;103(2):1059-1093. doi: 10.1152/physrev.00036.2021. Epub 2022 Oct 6. Physiol Rev. 2023. PMID: 36201635 Free PMC article. Review.
References
-
- Bracke KR, Verhamme FM, Seys LJ, Bantsimba-Malanda C, Cunoosamy DM, Herbst R, Hammad H, Lambrecht BN, Joos GF, Brusselle GG. Role of CXCL13 in cigarette smoke-induced lymphoid follicle formation and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:343–355. - PubMed
-
- Cyster JG. Lymphoid organ development and cell migration. Immunol Rev. 2003;195:5–14. - PubMed
-
- Demoor T, Bracke KR, Maes T, Vandooren B, Elewaut D, Pilette C, Joos GF, Brusselle GG. Role of lymphotoxin-alpha in cigarette smoke-induced inflammation and lymphoid neogenesis. Eur Respir J. 2009;34:405–416. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

