Physiologically activated mammary fibroblasts promote postpartum mammary cancer
- PMID: 28352652
- PMCID: PMC5358521
- DOI: 10.1172/jci.insight.89206
Physiologically activated mammary fibroblasts promote postpartum mammary cancer
Abstract
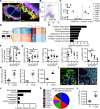
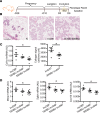
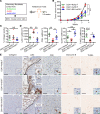
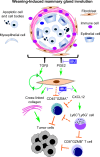
Women diagnosed with breast cancer within 5 years of childbirth have poorer prognosis than nulliparous or pregnant women. Weaning-induced breast involution is implicated, as the collagen-rich, immunosuppressive microenvironment of the involuting mammary gland is tumor promotional in mice. To investigate the role of mammary fibroblasts, isolated mammary PDGFRα+ cells from nulliparous and postweaning mice were assessed for activation phenotype and protumorigenic function. Fibroblast activation during involution was evident by increased expression of fibrillar collagens, lysyl oxidase, Tgfb1, and Cxcl12 genes. The ability of mammary tumors to grow in an isogenic, orthotopic transplant model was increased when tumor cells were coinjected with involution-derived compared with nulliparous-derived mammary fibroblasts. Mammary tumors in the involution-fibroblast group had increased Ly6C+ monocytes at the tumor border, and decreased CD8+ T cell infiltration and tumor cell death. Ibuprofen treatment suppressed involution-fibroblast activation and tumor promotional capacity, concurrent with decreases in tumor Ly6C+ monocytes, and increases in intratumoral CD8+ T cell infiltration, granzyme levels, and tumor cell death. In total, our data identify a COX/prostaglandin E2 (PGE2)-dependent activated mammary fibroblast within the involuting mammary gland that displays protumorigenic, immunosuppressive activity, identifying fibroblasts as potential targets for the prevention and treatment of postpartum breast cancer.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures


Similar articles
-
Non-steroidal anti-inflammatory drugs target the pro-tumorigenic extracellular matrix of the postpartum mammary gland.Int J Dev Biol. 2011;55(7-9):745-55. doi: 10.1387/ijdb.113379jo. Int J Dev Biol. 2011. PMID: 22161831
-
Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer.J Immunother Cancer. 2018 Oct 1;6(1):98. doi: 10.1186/s40425-018-0406-y. J Immunother Cancer. 2018. PMID: 30285905 Free PMC article.
-
Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species.Am J Pathol. 2010 Mar;176(3):1241-55. doi: 10.2353/ajpath.2010.090735. Epub 2010 Jan 28. Am J Pathol. 2010. PMID: 20110414 Free PMC article.
-
Mammary gland involution as an immunotherapeutic target for postpartum breast cancer.J Mammary Gland Biol Neoplasia. 2014 Jul;19(2):213-28. doi: 10.1007/s10911-014-9322-z. Epub 2014 Jun 22. J Mammary Gland Biol Neoplasia. 2014. PMID: 24952477 Free PMC article. Review.
-
Macphatics and PoEMs in Postpartum Mammary Development and Tumor Progression.J Mammary Gland Biol Neoplasia. 2020 Jun;25(2):103-113. doi: 10.1007/s10911-020-09451-6. Epub 2020 Jun 13. J Mammary Gland Biol Neoplasia. 2020. PMID: 32535810 Free PMC article. Review.
Cited by
-
Premenopausal women with breast cancer in the early post-partum period show molecular profiles of invasion and are associated with poor prognosis.Breast Cancer Res Treat. 2023 Jul;200(1):139-149. doi: 10.1007/s10549-023-06956-6. Epub 2023 May 9. Breast Cancer Res Treat. 2023. PMID: 37160509 Free PMC article.
-
Pregnancy and weaning regulate human maternal liver size and function.Proc Natl Acad Sci U S A. 2021 Nov 30;118(48):e2107269118. doi: 10.1073/pnas.2107269118. Proc Natl Acad Sci U S A. 2021. PMID: 34815335 Free PMC article.
-
Host response during unresolved urinary tract infection alters female mammary tissue homeostasis through collagen deposition and TIMP1.Nat Commun. 2024 Apr 16;15(1):3282. doi: 10.1038/s41467-024-47462-7. Nat Commun. 2024. PMID: 38627380 Free PMC article.
-
Postpartum breast cancer progression is driven by semaphorin 7a-mediated invasion and survival.Oncogene. 2020 Mar;39(13):2772-2785. doi: 10.1038/s41388-020-1192-9. Epub 2020 Feb 4. Oncogene. 2020. PMID: 32020054 Free PMC article.
-
Postpartum breast cancer has a distinct molecular profile that predicts poor outcomes.Nat Commun. 2021 Nov 3;12(1):6341. doi: 10.1038/s41467-021-26505-3. Nat Commun. 2021. PMID: 34732713 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

