Evaluation of methods for pore generation and their influence on physio-chemical properties of a protein based hydrogel
- PMID: 28352549
- PMCID: PMC5361077
- DOI: 10.1016/j.btre.2016.09.001
Evaluation of methods for pore generation and their influence on physio-chemical properties of a protein based hydrogel
Abstract
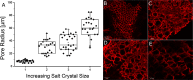
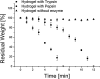
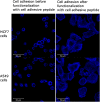
Different methods to create and manipulate pore sizes in hydrogel fabrication are available, but systematic studies are normally conducted with hydrogels made of synthetic chemical compounds as backbones. In this study, a hydrogel made of natural and abundant protein in combination with different, well-available techniques was used to produce different architectures within the hydrogel matrix. Pore sizes and distribution are compared and resulting hydrogel properties like swelling ratio, resistance towards external stimuli and enzymatic degradation were investigated. Porous hydrogels were functionalized and two cancer cell lines were successfully adhered onto the material. With simple methods, pores with a radius between 10 and 80 μm and channels of 25 μm radius with a length of several hundreds of μm could be created and analyzed with laser scanning confocal microscopy and electron microscopy respectively. Furthermore, the influence of different methods on swelling ratio, enzymatic degradation and pH and temperature resistance was observed.
Keywords: Biopolymers; Cell culture; Degradation; Hydrogel properties; Pore size.
Figures




Similar articles
-
Easy Manipulation of Architectures in Protein-based Hydrogels for Cell Culture Applications.J Vis Exp. 2017 Aug 4;(126):55813. doi: 10.3791/55813. J Vis Exp. 2017. PMID: 28809838 Free PMC article.
-
Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying.Acta Biomater. 2019 Aug;94:195-203. doi: 10.1016/j.actbio.2019.05.070. Epub 2019 May 30. Acta Biomater. 2019. PMID: 31154055
-
Fabrication and development of artificial osteochondral constructs based on cancellous bone/hydrogel hybrid scaffold.J Mater Sci Mater Med. 2016 Jun;27(6):114. doi: 10.1007/s10856-016-5722-5. Epub 2016 May 14. J Mater Sci Mater Med. 2016. PMID: 27180235
-
Natural Polymer-based Stimuli-responsive Hydrogels.Curr Med Chem. 2020;27(16):2631-2657. doi: 10.2174/0929867326666191122144916. Curr Med Chem. 2020. PMID: 31755377 Review.
-
Supramolecular Hydrogels Based on DNA Self-Assembly.Acc Chem Res. 2017 Apr 18;50(4):659-668. doi: 10.1021/acs.accounts.6b00524. Epub 2017 Mar 16. Acc Chem Res. 2017. PMID: 28299927 Review.
Cited by
-
Numerical Investigations of Hepatic Spheroids Metabolic Reactions in a Perfusion Bioreactor.Front Bioeng Biotechnol. 2019 Sep 12;7:221. doi: 10.3389/fbioe.2019.00221. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31572719 Free PMC article.
-
Easy Manipulation of Architectures in Protein-based Hydrogels for Cell Culture Applications.J Vis Exp. 2017 Aug 4;(126):55813. doi: 10.3791/55813. J Vis Exp. 2017. PMID: 28809838 Free PMC article.
-
The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives.Biomimetics (Basel). 2024 Apr 12;9(4):230. doi: 10.3390/biomimetics9040230. Biomimetics (Basel). 2024. PMID: 38667241 Free PMC article. Review.
-
Synthesis of nanocapsules blended polymeric hydrogel loaded with bupivacaine drug delivery system for local anesthetics and pain management.Drug Deliv. 2022 Dec;29(1):399-412. doi: 10.1080/10717544.2021.2023702. Drug Deliv. 2022. PMID: 35098821 Free PMC article.
-
Fabrication and Evaluation of Silk Sericin-Derived Hydrogel for the Release of the Model Drug Berberine.Gels. 2021 Feb 20;7(1):23. doi: 10.3390/gels7010023. Gels. 2021. PMID: 33672687 Free PMC article.
References
-
- Raic A., Rödling L., Kalbacher H., Lee-Thedieck C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 2014;35:929–940. - PubMed
-
- Sasaki J.-I., Asoh T.-A., Matsumoto T., Egusa H., Sohmura T., Alsberg E., Akashi M., Yatani H. Fabrication of three-dimensional cell constructs using temperature-responsive hydrogel. Tissue Eng. Part A. 2010;16:2497–2504. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
