High-throughput identification of small molecules that affect human embryonic vascular development
- PMID: 28348206
- PMCID: PMC5393190
- DOI: 10.1073/pnas.1617451114
High-throughput identification of small molecules that affect human embryonic vascular development
Abstract
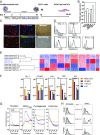
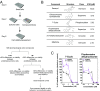
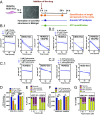
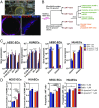
Birth defects, which are in part caused by exposure to environmental chemicals and pharmaceutical drugs, affect 1 in every 33 babies born in the United States each year. The current standard to screen drugs that affect embryonic development is based on prenatal animal testing; however, this approach yields low-throughput and limited mechanistic information regarding the biological pathways and potential adverse consequences in humans. To develop a screening platform for molecules that affect human embryonic development based on endothelial cells (ECs) derived from human pluripotent stem cells, we differentiated human pluripotent stem cells into embryonic ECs and induced their maturation under arterial flow conditions. These cells were then used to screen compounds that specifically affect embryonic vasculature. Using this platform, we have identified two compounds that have higher inhibitory effect in embryonic than postnatal ECs. One of them was fluphenazine (an antipsychotic), which inhibits calmodulin kinase II. The other compound was pyrrolopyrimidine (an antiinflammatory agent), which inhibits vascular endothelial growth factor receptor 2 (VEGFR2), decreases EC viability, induces an inflammatory response, and disrupts preformed vascular networks. The vascular effect of the pyrrolopyrimidine was further validated in prenatal vs. adult mouse ECs and in embryonic and adult zebrafish. We developed a platform based on human pluripotent stem cell-derived ECs for drug screening, which may open new avenues of research for the study and modulation of embryonic vasculature.
Keywords: embryonic endothelial markers; endothelial cells; high-throughput screening; pluripotent stem cells; vascular toxicity.
Conflict of interest statement
Conflict of interest statement: A patent has been filed for this work (“Differentiated Cell Population of Endothelial Cells Derived from Human Pluripotent Stem Cells, Composition System, Kit and Uses Therefore”; PCT/IB2013/061110).
Figures
Similar articles
-
Angiopoietin-1 promotes endothelial differentiation from embryonic stem cells and induced pluripotent stem cells.Blood. 2011 Aug 25;118(8):2094-104. doi: 10.1182/blood-2010-12-323907. Epub 2011 Jun 16. Blood. 2011. PMID: 21680798
-
Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts.Arterioscler Thromb Vasc Biol. 2014 Jan;34(1):177-86. doi: 10.1161/ATVBAHA.113.302598. Epub 2013 Oct 24. Arterioscler Thromb Vasc Biol. 2014. PMID: 24158517
-
Human Induced Pluripotent Stem Cell-Derived Cardiac Progenitor Cells in Phenotypic Screening: A Transforming Growth Factor-β Type 1 Receptor Kinase Inhibitor Induces Efficient Cardiac Differentiation.Stem Cells Transl Med. 2016 Feb;5(2):164-74. doi: 10.5966/sctm.2015-0114. Epub 2015 Dec 18. Stem Cells Transl Med. 2016. PMID: 26683871 Free PMC article.
-
Molecular pathways governing development of vascular endothelial cells from ES/iPS cells.Stem Cell Rev Rep. 2013 Oct;9(5):586-98. doi: 10.1007/s12015-013-9450-7. Stem Cell Rev Rep. 2013. PMID: 23765563 Review.
-
MicroRNA regulation of endothelial homeostasis and commitment-implications for vascular regeneration strategies using stem cell therapies.Free Radic Biol Med. 2013 Sep;64:52-60. doi: 10.1016/j.freeradbiomed.2013.04.037. Epub 2013 May 9. Free Radic Biol Med. 2013. PMID: 23665307 Review.
Cited by
-
Human Engineered Heart Tissue Models for Disease Modeling and Drug Discovery.Front Cell Dev Biol. 2022 Mar 31;10:855763. doi: 10.3389/fcell.2022.855763. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35433691 Free PMC article. Review.
-
[Leukocyte count of puerperal sows].Berl Munch Tierarztl Wochenschr. 1996 Sep;109(9):330-5. Berl Munch Tierarztl Wochenschr. 1996. PMID: 9054332 Free PMC article.
-
Blockade of vascular endothelial growth factor receptor 2 inhibits intraplaque haemorrhage by normalization of plaque neovessels.J Intern Med. 2019 Jan;285(1):59-74. doi: 10.1111/joim.12821. Epub 2018 Sep 7. J Intern Med. 2019. PMID: 30102798 Free PMC article.
-
Human iPSC modeling of heart disease for drug development.Cell Chem Biol. 2021 Mar 18;28(3):271-282. doi: 10.1016/j.chembiol.2021.02.016. Cell Chem Biol. 2021. PMID: 33740432 Free PMC article. Review.
-
Inflammatory Responses and Barrier Function of Endothelial Cells Derived from Human Induced Pluripotent Stem Cells.Stem Cell Reports. 2018 May 8;10(5):1642-1656. doi: 10.1016/j.stemcr.2018.03.012. Epub 2018 Apr 12. Stem Cell Reports. 2018. PMID: 29657098 Free PMC article.
References
-
- Makris SL, et al. Current and future needs for developmental toxicity testing. Birth Defects Res B Dev Reprod Toxicol. 2011;92(5):384–394. - PubMed
-
- Vasvari G, Dyckhoff G, Herold-Mende C. Thalidomide protects endothelial cells from doxorubicin-induced apoptosis but alters cell morphology—a rebuttal. J Thromb Haemost. 2005;3(4):816–817, author reply 817–818. - PubMed
-
- Knudsen TB, Kleinstreuer NC. Disruption of embryonic vascular development in predictive toxicology. Birth Defects Res C Embryo Today. 2011;93(4):312–323. - PubMed
-
- Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med. 1962;267:1184–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

