Metabolic Impact of Light Phase-Restricted Fructose Consumption Is Linked to Changes in Hypothalamic AMPK Phosphorylation and Melatonin Production in Rats
- PMID: 28346369
- PMCID: PMC5409671
- DOI: 10.3390/nu9040332
Metabolic Impact of Light Phase-Restricted Fructose Consumption Is Linked to Changes in Hypothalamic AMPK Phosphorylation and Melatonin Production in Rats
Abstract
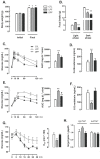
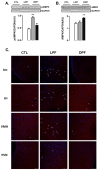
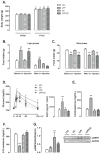
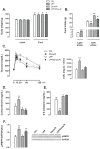
Recent studies show that the metabolic effects of fructose may vary depending on the phase of its consumption along with the light/dark cycle. Here, we investigated the metabolic outcomes of fructose consumption by rats during either the light (LPF) or the dark (DPF) phases of the light/dark cycle. This experimental approach was combined with other interventions, including restriction of chow availability to the dark phase, melatonin administration or intracerebroventricular inhibition of adenosine monophosphate-activated protein kinase (AMPK) with Compound C. LPF, but not DPF rats, exhibited increased hypothalamic AMPK phosphorylation, glucose intolerance, reduced urinary 6-sulfatoxymelatonin (6-S-Mel) (a metabolite of melatonin) and increased corticosterone levels. LPF, but not DPF rats, also exhibited increased chow ingestion during the light phase. The mentioned changes were blunted by Compound C. LPF rats subjected to dark phase-restricted feeding still exhibited increased hypothalamic AMPK phosphorylation but failed to develop the endocrine and metabolic changes. Moreover, melatonin administration to LPF rats reduced corticosterone and prevented glucose intolerance. Altogether, the present data suggests that consumption of fructose during the light phase results in out-of-phase feeding due to increased hypothalamic AMPK phosphorylation. This shift in spontaneous chow ingestion is responsible for the reduction of 6-S-Mel and glucose intolerance.
Keywords: AMPK; corticosterone; fructose; melatonin; out-of-phase feeding.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise, associated with this article. The authors are responsible for the writing and content of the article.
Figures
Similar articles
-
Fructose-induced hypothalamic AMPK activation stimulates hepatic PEPCK and gluconeogenesis due to increased corticosterone levels.Endocrinology. 2012 Aug;153(8):3633-45. doi: 10.1210/en.2012-1341. Epub 2012 May 14. Endocrinology. 2012. PMID: 22585831
-
Olanzapine induces glucose intolerance through the activation of AMPK in the mouse hypothalamus.Eur J Pharmacol. 2013 Oct 15;718(1-3):376-82. doi: 10.1016/j.ejphar.2013.08.006. Epub 2013 Aug 22. Eur J Pharmacol. 2013. PMID: 23973646
-
Liver AMP/ATP ratio and fructokinase expression are related to gender differences in AMPK activity and glucose intolerance in rats ingesting liquid fructose.J Nutr Biochem. 2011 Aug;22(8):741-51. doi: 10.1016/j.jnutbio.2010.06.005. Epub 2010 Nov 5. J Nutr Biochem. 2011. PMID: 21115336
-
Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats.J Nutr Biochem. 2014 Apr;25(4):420-8. doi: 10.1016/j.jnutbio.2013.11.014. Epub 2013 Dec 31. J Nutr Biochem. 2014. PMID: 24491314
-
Light, Water, and Melatonin: The Synergistic Regulation of Phase Separation in Dementia.Int J Mol Sci. 2023 Mar 19;24(6):5835. doi: 10.3390/ijms24065835. Int J Mol Sci. 2023. PMID: 36982909 Free PMC article. Review.
Cited by
-
Alterations of proteome, mitochondrial dynamic and autophagy in the hypothalamus during activity-based anorexia.Sci Rep. 2018 May 8;8(1):7233. doi: 10.1038/s41598-018-25548-9. Sci Rep. 2018. PMID: 29740148 Free PMC article.
-
In Utero Dexamethasone Exposure Exacerbates Hepatic Steatosis in Rats That Consume Fructose During Adulthood.Nutrients. 2019 Sep 5;11(9):2114. doi: 10.3390/nu11092114. Nutrients. 2019. PMID: 31491968 Free PMC article.
-
Fructose Consumption by Adult Rats Exposed to Dexamethasone In Utero Changes the Phenotype of Intestinal Epithelial Cells and Exacerbates Intestinal Gluconeogenesis.Nutrients. 2020 Oct 7;12(10):3062. doi: 10.3390/nu12103062. Nutrients. 2020. PMID: 33036430 Free PMC article.
References
-
- Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L., Hatcher B., Cox C.L., Dyachenko A., Zhang W., et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009;119:1322–1334. doi: 10.1172/JCI37385. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

