Type I Interferons as Regulators of Lung Inflammation
- PMID: 28344581
- PMCID: PMC5344902
- DOI: 10.3389/fimmu.2017.00259
Type I Interferons as Regulators of Lung Inflammation
Abstract
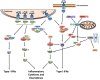
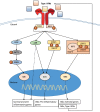
Immune responses to lung infections must be tightly regulated in order to permit pathogen eradication while maintaining organ function. Exuberant or dysregulated inflammation can impair gas exchange and underlies many instances of lung disease. An important driver of inflammation in the lung is the interferon (IFN) response. Type I IFNs are antiviral cytokines that induce a large range of proteins that impair viral replication in infected cells. This cell-intrinsic action plays a crucial role in protecting the lungs from spread of respiratory viruses. However, type I IFNs have also recently been found to be central to the initiation of lung inflammatory responses, by inducing recruitment and activation of immune cells. This helps control virus burden but can cause detrimental immunopathology and contribute to disease severity. Furthermore, there is now increasing evidence that type I IFNs are not only induced after viral infections but also after infection with bacteria and fungi. The pro-inflammatory function of type I IFNs in the lung opens up the possibility of immune modulation directed against this antiviral cytokine family. In this review, the initiation and signaling of type I IFNs as well as their role in driving and maintaining lung inflammation will be discussed.
Keywords: infection; inflammation; lung; pattern recognition receptors; type I interferons.
Figures
Similar articles
-
Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection.J Virol. 2014 Jun;88(11):6128-36. doi: 10.1128/JVI.00333-14. Epub 2014 Mar 19. J Virol. 2014. PMID: 24648449 Free PMC article.
-
Functional Interplay between Type I and II Interferons Is Essential to Limit Influenza A Virus-Induced Tissue Inflammation.PLoS Pathog. 2016 Jan 5;12(1):e1005378. doi: 10.1371/journal.ppat.1005378. eCollection 2016 Jan. PLoS Pathog. 2016. PMID: 26731100 Free PMC article.
-
Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling.J Virol. 2018 Jan 30;92(4):e01677-17. doi: 10.1128/JVI.01677-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187542 Free PMC article.
-
Induction and regulation of IFNs during viral infections.J Interferon Cytokine Res. 2004 Aug;24(8):439-54. doi: 10.1089/1079990041689665. J Interferon Cytokine Res. 2004. PMID: 15320958 Review.
-
Type I and III interferon production in response to RNA viruses.J Interferon Cytokine Res. 2014 Sep;34(9):649-58. doi: 10.1089/jir.2014.0066. Epub 2014 Jun 23. J Interferon Cytokine Res. 2014. PMID: 24956361 Review.
Cited by
-
Longitudinal Analysis of Urinary Cytokines and Biomarkers in COVID-19 Patients with Subclinical Acute Kidney Injury.Int J Mol Sci. 2022 Dec 6;23(23):15419. doi: 10.3390/ijms232315419. Int J Mol Sci. 2022. PMID: 36499745 Free PMC article.
-
Transcriptomics in lung tissue upon respiratory syncytial virus infection reveals aging as important modulator of immune activation and matrix maintenance.Sci Rep. 2018 Nov 9;8(1):16653. doi: 10.1038/s41598-018-35180-2. Sci Rep. 2018. PMID: 30413794 Free PMC article.
-
Immune response to SARS-CoV-2 in severe disease and long COVID-19.iScience. 2022 Aug 19;25(8):104723. doi: 10.1016/j.isci.2022.104723. Epub 2022 Jul 4. iScience. 2022. PMID: 35813874 Free PMC article.
-
Interplay between SARS-CoV-2 and the type I interferon response.PLoS Pathog. 2020 Jul 29;16(7):e1008737. doi: 10.1371/journal.ppat.1008737. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32726355 Free PMC article. Review.
-
Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection.Viruses. 2019 Jan 15;11(1):62. doi: 10.3390/v11010062. Viruses. 2019. PMID: 30650519 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

