Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies
- PMID: 28336552
- PMCID: PMC5482929
- DOI: 10.1158/2159-8290.CD-16-1337
Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies
Abstract
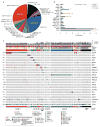
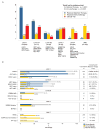
Tumor genetic testing is standard of care for patients with advanced lung adenocarcinoma, but the fraction of patients who derive clinical benefit remains undefined. Here, we report the experience of 860 patients with metastatic lung adenocarcinoma analyzed prospectively for mutations in >300 cancer-associated genes. Potentially actionable genetic events were stratified into one of four levels based upon published clinical or laboratory evidence that the mutation in question confers increased sensitivity to standard or investigational therapies. Overall, 37.1% (319/860) of patients received a matched therapy guided by their tumor molecular profile. Excluding alterations associated with standard-of-care therapy, 14.4% (69/478) received matched therapy, with a clinical benefit of 52%. Use of matched therapy was strongly influenced by the level of preexistent clinical evidence that the mutation identified predicts for drug response. Analysis of genes mutated significantly more often in tumors without known actionable mutations nominated STK11 and KEAP1 as possible targetable mitogenic drivers.Significance: An increasing number of therapies that target molecular alterations required for tumor maintenance and progression have demonstrated clinical activity in patients with lung adenocarcinoma. The data reported here suggest that broader, early testing for molecular alterations that have not yet been recognized as standard-of-care predictive biomarkers of drug response could accelerate the development of targeted agents for rare mutational events and could result in improved clinical outcomes. Cancer Discov; 7(6); 596-609. ©2017 AACR.See related commentary by Liu et al., p. 555This article is highlighted in the In This Issue feature, p. 539.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures


Comment in
-
Toward Molecularly Driven Precision Medicine in Lung Adenocarcinoma.Cancer Discov. 2017 Jun;7(6):555-557. doi: 10.1158/2159-8290.CD-17-0355. Cancer Discov. 2017. PMID: 28576842 Free PMC article.
Similar articles
-
Toward Molecularly Driven Precision Medicine in Lung Adenocarcinoma.Cancer Discov. 2017 Jun;7(6):555-557. doi: 10.1158/2159-8290.CD-17-0355. Cancer Discov. 2017. PMID: 28576842 Free PMC article.
-
Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches.Ther Adv Respir Dis. 2016 Apr;10(2):113-29. doi: 10.1177/1753465815617871. Epub 2015 Nov 30. Ther Adv Respir Dis. 2016. PMID: 26620497 Free PMC article. Review.
-
High Prevalence of Concomitant Oncogene Mutations in Prospectively Identified Patients with ROS1-Positive Metastatic Lung Cancer.J Thorac Oncol. 2017 Jan;12(1):54-64. doi: 10.1016/j.jtho.2016.08.137. Epub 2016 Aug 27. J Thorac Oncol. 2017. PMID: 27575422
-
Genomic profiling of lung adenocarcinoma patients reveals therapeutic targets and confers clinical benefit when standard molecular testing is negative.Oncotarget. 2016 Apr 26;7(17):24172-8. doi: 10.18632/oncotarget.8138. Oncotarget. 2016. PMID: 26992220 Free PMC article.
-
Molecular genetic testing for lung adenocarcinomas: a practical approach to clinically relevant mutations and translocations.J Clin Pathol. 2013 Oct;66(10):870-4. doi: 10.1136/jclinpath-2012-201336. Epub 2013 Jun 25. J Clin Pathol. 2013. PMID: 23801495 Review.
Cited by
-
Real-world utility of next-generation sequencing for targeted gene analysis and its application to treatment in lung adenocarcinoma.Cancer Med. 2021 May;10(10):3197-3204. doi: 10.1002/cam4.3874. Epub 2021 May 7. Cancer Med. 2021. PMID: 33960703 Free PMC article.
-
The current state of the art and future trends in RAS-targeted cancer therapies.Nat Rev Clin Oncol. 2022 Oct;19(10):637-655. doi: 10.1038/s41571-022-00671-9. Epub 2022 Aug 26. Nat Rev Clin Oncol. 2022. PMID: 36028717 Free PMC article. Review.
-
High frequency of exon 20 S768I EGFR mutation detected in malignant pleural effusions: A poor prognosticator of NSCLC.Cancer Rep (Hoboken). 2020 Oct;3(5):e1262. doi: 10.1002/cnr2.1262. Epub 2020 Aug 6. Cancer Rep (Hoboken). 2020. PMID: 32761886 Free PMC article.
-
Multi-Omics Mining of lncRNAs with Biological and Clinical Relevance in Cancer.Int J Mol Sci. 2023 Nov 22;24(23):16600. doi: 10.3390/ijms242316600. Int J Mol Sci. 2023. PMID: 38068923 Free PMC article. Review.
-
DSTYK inhibition increases the sensitivity of lung cancer cells to T cell-mediated cytotoxicity.J Exp Med. 2022 Dec 5;219(12):e20220726. doi: 10.1084/jem.20220726. Epub 2022 Sep 28. J Exp Med. 2022. PMID: 36169652 Free PMC article.
References
-
- Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. The New England journal of medicine. 2013;368(25):2385–94. - PubMed
-
- Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. The New England journal of medicine. 2014;371(23):2167–77. - PubMed
-
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology. 2012;13(3):239–46. - PubMed
-
- Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431(7008):525–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

