Aberrant iPSC-derived human astrocytes in Alzheimer's disease
- PMID: 28333144
- PMCID: PMC5386580
- DOI: 10.1038/cddis.2017.89
Aberrant iPSC-derived human astrocytes in Alzheimer's disease
Erratum in
-
Correction: Aberrant iPSC-derived human astrocytes in Alzheimer's disease.Cell Death Dis. 2019 Mar 12;10(3):244. doi: 10.1038/s41419-019-1422-7. Cell Death Dis. 2019. PMID: 30862780 Free PMC article.
Abstract
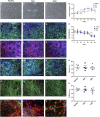
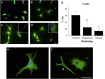
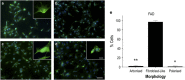
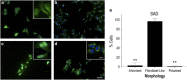
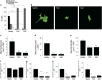
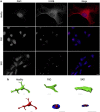
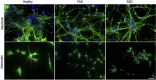
The pathological potential of human astroglia in Alzheimer's disease (AD) was analysed in vitro using induced pluripotent stem cell (iPSC) technology. Here, we report development of a human iPSC-derived astrocyte model created from healthy individuals and patients with either early-onset familial AD (FAD) or the late-onset sporadic form of AD (SAD). Our chemically defined and highly efficient model provides >95% homogeneous populations of human astrocytes within 30 days of differentiation from cortical neural progenitor cells (NPCs). All astrocytes expressed functional markers including glial fibrillary acidic protein (GFAP), excitatory amino acid transporter-1 (EAAT1), S100B and glutamine synthetase (GS) comparable to that of adult astrocytes in vivo. However, induced astrocytes derived from both SAD and FAD patients exhibit a pronounced pathological phenotype, with a significantly less complex morphological appearance, overall atrophic profiles and abnormal localisation of key functional astroglial markers. Furthermore, NPCs derived from identical patients did not show any differences, therefore, validating that remodelled astroglia are not as a result of defective neural intermediates. This work not only presents a novel model to study the mechanisms of human astrocytes in vitro, but also provides an ideal platform for further interrogation of early astroglial cell autonomous events in AD and the possibility of identification of novel therapeutic targets for the treatment of AD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Glutamine synthetase in astrocytes from entorhinal cortex of the triple transgenic animal model of Alzheimer's disease is not affected by pathological progression.Biogerontology. 2013 Dec;14(6):777-87. doi: 10.1007/s10522-013-9456-1. Epub 2013 Aug 30. Biogerontology. 2013. PMID: 23990215
-
Prominent and conspicuous astrocyte atrophy in human sporadic and familial Alzheimer's disease.Brain Struct Funct. 2023 Dec;228(9):2103-2113. doi: 10.1007/s00429-023-02707-x. Epub 2023 Sep 20. Brain Struct Funct. 2023. PMID: 37730895 Free PMC article.
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
Modelling Sporadic Alzheimer's Disease Using Induced Pluripotent Stem Cells.Neurochem Res. 2018 Dec;43(12):2179-2198. doi: 10.1007/s11064-018-2663-z. Epub 2018 Nov 1. Neurochem Res. 2018. PMID: 30387070 Free PMC article. Review.
-
Human Pluripotent Stem Cell-Derived Neural Cells as a Relevant Platform for Drug Screening in Alzheimer's Disease.Int J Mol Sci. 2020 Sep 18;21(18):6867. doi: 10.3390/ijms21186867. Int J Mol Sci. 2020. PMID: 32962164 Free PMC article. Review.
Cited by
-
Inflammatory aspects of Alzheimer's disease.Acta Neuropathol. 2024 Aug 28;148(1):31. doi: 10.1007/s00401-024-02790-2. Acta Neuropathol. 2024. PMID: 39196440 Review.
-
Quantitative Systems Pharmacology for Neuroscience Drug Discovery and Development: Current Status, Opportunities, and Challenges.CPT Pharmacometrics Syst Pharmacol. 2020 Jan;9(1):5-20. doi: 10.1002/psp4.12478. Epub 2019 Nov 24. CPT Pharmacometrics Syst Pharmacol. 2020. PMID: 31674729 Free PMC article.
-
Molecular Insights into Cell Type-specific Roles in Alzheimer's Disease: Human Induced Pluripotent Stem Cell-based Disease Modelling.Neuroscience. 2023 May 10;518:10-26. doi: 10.1016/j.neuroscience.2022.05.006. Epub 2022 May 13. Neuroscience. 2023. PMID: 35569647 Free PMC article. Review.
-
Modeling Alzheimer's disease with iPSC-derived brain cells.Mol Psychiatry. 2020 Jan;25(1):148-167. doi: 10.1038/s41380-019-0468-3. Epub 2019 Aug 7. Mol Psychiatry. 2020. PMID: 31391546 Free PMC article. Review.
-
Astrocytes in Alzheimer's Disease: Pathological Significance and Molecular Pathways.Cells. 2021 Mar 4;10(3):540. doi: 10.3390/cells10030540. Cells. 2021. PMID: 33806259 Free PMC article. Review.
References
-
- Verkhratsky A, Butt AM Glial Physiology and Pathophysiology. Wiley-Blackwell: Chichester, UK, 2013.
-
- Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol 2016; 131: 323–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

