γ-Tocotrienol Inhibits TGF-β1-Induced Contractile Phenotype Expression of Human Airway Smooth Muscle Cells
- PMID: 28331417
- PMCID: PMC5355840
γ-Tocotrienol Inhibits TGF-β1-Induced Contractile Phenotype Expression of Human Airway Smooth Muscle Cells
Abstract
Background: Tocotrienols, members of the vitamin E family, exist in four different isoforms (α, β, γ and δ tocotrienol) that have can be protective against brain damage, as well as having anticancer effects in vivo and in vitro. We have shown that γ-tocotrienol inhibits human airway smooth muscle cell proliferation and migration induced by platelet-derived growth factor (PDGF)-BB by suppressing RhoA activation. In this study, we tested whether γ-tocotrienol modulates transforming growth factor (TGF) -β-induced induction of human airway smooth muscle (ASM) into a contractile phenotype and concomitant synthesis of extracellular matrix proteins.
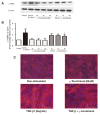
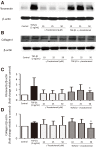
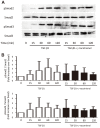
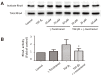
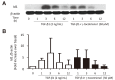
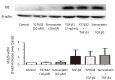
Methods: ASM cells were stimulated with TGF-β1 (2 ng/mL) for 48 hours and the effect of γ-tocotrienol (50 μM) on α-smooth muscle actin, fibronectin and collagen I expression was assessed using Western blotting. The signaling pathways involved in TGF-β1 stimulation were also investigated.
Results: TGF-β1 increased α-smooth muscle actin, fibronectin and collagen Ⅰ abundance by 3- to 5-fold. This response was inhibited significantly by γ-tocotrienol. Furthermore, γ-tocotrienol suppressed RhoA activation, but did not affect Smad2 or Smad3 phosphorylation.
Conclusion: These results indicate that γ-tocotrienol has potential for benefit in modulating on airway remodeling in asthma, likely via a mechanism involving the suppression of TGF-β activation of RhoA.
Keywords: airway smooth muscle; asthma; remodeling; vitamin E; γ-Tocotrienol.
Figures
Similar articles
-
β-Tocotrienol Decreases PDGF-BB-Induced Proliferation and Migration of Human Airway Smooth Muscle Cells by Inhibiting RhoA and Reducing ROS Production.Pharmaceuticals (Basel). 2024 May 30;17(6):712. doi: 10.3390/ph17060712. Pharmaceuticals (Basel). 2024. PMID: 38931379 Free PMC article.
-
γ-Tocotrienol reduces human airway smooth muscle cell proliferation and migration.Pulm Pharmacol Ther. 2015 Jun;32:45-52. doi: 10.1016/j.pupt.2015.04.003. Epub 2015 May 6. Pulm Pharmacol Ther. 2015. PMID: 25956071
-
A HuR/TGF-β1 feedback circuit regulates airway remodeling in airway smooth muscle cells.Respir Res. 2016 Sep 22;17(1):117. doi: 10.1186/s12931-016-0437-1. Respir Res. 2016. PMID: 27658983 Free PMC article.
-
Protocatechuic acid inhibits TGF-β1-induced proliferation and migration of human airway smooth muscle cells.J Pharmacol Sci. 2019 Jan;139(1):9-14. doi: 10.1016/j.jphs.2018.10.011. Epub 2018 Nov 5. J Pharmacol Sci. 2019. PMID: 30472056
-
L-thyroxine promotes a proliferative airway smooth muscle phenotype in the presence of TGF-β1.Am J Physiol Lung Cell Mol Physiol. 2015 Feb 1;308(3):L301-6. doi: 10.1152/ajplung.00071.2014. Epub 2014 Dec 5. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25480330
Cited by
-
Anti-inflammatory Activity of Tocotrienols in Age-related Pathologies: A SASPected Involvement of Cellular Senescence.Biol Proced Online. 2018 Nov 20;20:22. doi: 10.1186/s12575-018-0087-4. eCollection 2018. Biol Proced Online. 2018. PMID: 30479579 Free PMC article. Review.
-
Blood Inflammatory-like and Lung Resident-like Eosinophils Affect Migration of Airway Smooth Muscle Cells and Their ECM-Related Proliferation in Asthma.Int J Mol Sci. 2023 Feb 9;24(4):3469. doi: 10.3390/ijms24043469. Int J Mol Sci. 2023. PMID: 36834879 Free PMC article.
-
β-Tocotrienol Decreases PDGF-BB-Induced Proliferation and Migration of Human Airway Smooth Muscle Cells by Inhibiting RhoA and Reducing ROS Production.Pharmaceuticals (Basel). 2024 May 30;17(6):712. doi: 10.3390/ph17060712. Pharmaceuticals (Basel). 2024. PMID: 38931379 Free PMC article.
-
TGF-β1 Evokes Human Airway Smooth Muscle Cell Shortening and Hyperresponsiveness via Smad3.Am J Respir Cell Mol Biol. 2018 May;58(5):575-584. doi: 10.1165/rcmb.2017-0247OC. Am J Respir Cell Mol Biol. 2018. PMID: 28984468 Free PMC article.
References
-
- Clarke DL, Dakshinamurti S, Larsson AK, Ward JE, Yamasaki A. Lipid metabolites as regulators of airway smooth muscle function. Pulm Pharmacol Ther. 2009;22:426-35. - PubMed
-
- Finiasz M, Otero C, Bezrodnik L, Fink S. The role of cytokines in atopic asthma. Curr Med Chem. 2011;18:1476-87. - PubMed
-
- Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor beta and severe asthma: a perfect storm. Respir Med. 2014;108:1409-23. - PubMed
-
- Burgess JK, Ceresa C, Johnson SR, Kanabar V, Moir LM, Nguyen TT, et al. Tissue and matrix influences on airway smooth muscle function. Pulm Pharmacol Ther. 2009;22:379-87. - PubMed
LinkOut - more resources
Full Text Sources
