Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury
- PMID: 28330995
- PMCID: PMC5389309
- DOI: 10.1073/pnas.1618291114
Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury
Abstract
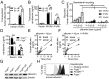
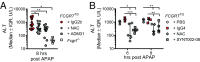
The neonatal crystallizable fragment receptor (FcRn) is responsible for maintaining the long half-life and high levels of the two most abundant circulating proteins, albumin and IgG. In the latter case, the protective mechanism derives from FcRn binding to IgG in the weakly acidic environment contained within endosomes of hematopoietic and parenchymal cells, whereupon IgG is diverted from degradation in lysosomes and is recycled. The cellular location and mechanism by which FcRn protects albumin are partially understood. Here we demonstrate that mice with global or liver-specific FcRn deletion exhibit hypoalbuminemia, albumin loss into the bile, and increased albumin levels in the hepatocyte. In vitro models with polarized cells illustrate that FcRn mediates basal recycling and bidirectional transcytosis of albumin and uniquely determines the physiologic release of newly synthesized albumin into the basal milieu. These properties allow hepatic FcRn to mediate albumin delivery and maintenance in the circulation, but they also enhance sensitivity to the albumin-bound hepatotoxin, acetaminophen (APAP). As such, global or liver-specific deletion of FcRn results in resistance to APAP-induced liver injury through increased albumin loss into the bile and increased intracellular albumin scavenging of reactive oxygen species. Further, protection from injury is achieved by pharmacologic blockade of FcRn-albumin interactions with monoclonal antibodies or peptide mimetics, which cause hypoalbuminemia, biliary loss of albumin, and increased intracellular accumulation of albumin in the hepatocyte. Together, these studies demonstrate that the main function of hepatic FcRn is to direct albumin into the circulation, thereby also increasing hepatocyte sensitivity to toxicity.
Keywords: FcRn; albumin; bile; liver; toxin.
Conflict of interest statement
Conflict of interest statement: R.S.B., W.I.L., D.C.R., and I.S. serve as consultants to Syntimmune, Inc., which is developing therapeutic agents directed at FcRn.
Figures

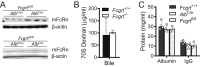
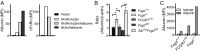




Similar articles
-
Half-life-extended recombinant coagulation factor IX-albumin fusion protein is recycled via the FcRn-mediated pathway.J Biol Chem. 2018 Apr 27;293(17):6363-6373. doi: 10.1074/jbc.M117.817064. Epub 2018 Mar 9. J Biol Chem. 2018. PMID: 29523681 Free PMC article.
-
N-Glycan Moieties in Neonatal Fc Receptor Determine Steady-state Membrane Distribution and Directional Transport of IgG.J Biol Chem. 2009 Mar 27;284(13):8292-300. doi: 10.1074/jbc.M805877200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19164298 Free PMC article.
-
Autophagy gene ATG7 regulates albumin transcytosis in renal tubule epithelial cells.Am J Physiol Renal Physiol. 2021 Nov 1;321(5):F572-F586. doi: 10.1152/ajprenal.00172.2021. Epub 2021 Sep 20. Am J Physiol Renal Physiol. 2021. PMID: 34541900
-
The immunologic functions of the neonatal Fc receptor for IgG.J Clin Immunol. 2013 Jan;33 Suppl 1(Suppl 1):S9-17. doi: 10.1007/s10875-012-9768-y. Epub 2012 Sep 5. J Clin Immunol. 2013. PMID: 22948741 Free PMC article. Review.
-
A Humanized Mouse Model to Study Human Albumin and Albumin Conjugates Pharmacokinetics.Methods Mol Biol. 2016;1438:115-22. doi: 10.1007/978-1-4939-3661-8_7. Methods Mol Biol. 2016. PMID: 27150087 Review.
Cited by
-
Circulating liver function markers and colorectal cancer risk: A prospective cohort study in the UK Biobank.Int J Cancer. 2021 Apr 15;148(8):1867-1878. doi: 10.1002/ijc.33351. Epub 2020 Nov 2. Int J Cancer. 2021. PMID: 33091956 Free PMC article.
-
Contribution of DNA methylation to the expression of FCGRT in human liver and myocardium.Sci Rep. 2019 Jun 17;9(1):8674. doi: 10.1038/s41598-019-45203-1. Sci Rep. 2019. PMID: 31209240 Free PMC article.
-
Neonatal Fc Receptor (FcRn) Enhances Tissue Distribution and Prevents Excretion of nab-Paclitaxel.Mol Pharm. 2019 Jun 3;16(6):2385-2393. doi: 10.1021/acs.molpharmaceut.8b01314. Epub 2019 May 1. Mol Pharm. 2019. PMID: 31002261 Free PMC article.
-
Deciphering the Dynamic Complexities of the Liver Microenvironment - Toward a Better Understanding of Immune-Mediated liver Injury Caused by Immune Checkpoint Inhibitors (ILICI).AAPS J. 2021 Aug 16;23(5):99. doi: 10.1208/s12248-021-00629-2. AAPS J. 2021. PMID: 34401948 Review.
-
Human and mouse albumin bind their respective neonatal Fc receptors differently.Sci Rep. 2018 Oct 2;8(1):14648. doi: 10.1038/s41598-018-32817-0. Sci Rep. 2018. PMID: 30279529 Free PMC article.
References
-
- Roopenian DC, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170(7):3528–3533. - PubMed
-
- Raghavan M, Bonagura VR, Morrison SL, Bjorkman PJ. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry. 1995;34(45):14649–14657. - PubMed
-
- Ward ES, Zhou J, Ghetie V, Ober RJ. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol. 2003;15(2):187–195. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

