Effects of Topical Icing on Inflammation, Angiogenesis, Revascularization, and Myofiber Regeneration in Skeletal Muscle Following Contusion Injury
- PMID: 28326040
- PMCID: PMC5339266
- DOI: 10.3389/fphys.2017.00093
Effects of Topical Icing on Inflammation, Angiogenesis, Revascularization, and Myofiber Regeneration in Skeletal Muscle Following Contusion Injury
Abstract
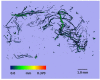
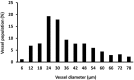
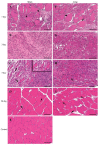
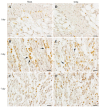
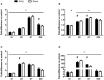
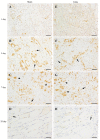
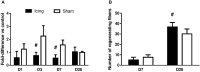
Contusion injuries in skeletal muscle commonly occur in contact sport and vehicular and industrial workplace accidents. Icing has traditionally been used to treat such injuries under the premise that it alleviates pain, reduces tissue metabolism, and modifies vascular responses to decrease swelling. Previous research has examined the effects of icing on inflammation and microcirculatory dynamics following muscle injury. However, whether icing influences angiogenesis, collateral vessel growth, or myofiber regeneration remains unknown. We compared the effects of icing vs. a sham treatment on the presence of neutrophils and macrophages; expression of CD34, von Willebrands factor (vWF), vascular endothelial growth factor (VEGF), and nestin; vessel volume; capillary density; and myofiber regeneration in skeletal after muscle contusion injury in rats. Muscle tissue was collected 1, 3, 7, and 28 d after injury. Compared with uninjured rats, muscles in rats that sustained the contusion injury exhibited major necrosis, inflammation, and increased expression of CD34, vWF, VEGF, and nestin. Compared with the sham treatment, icing attenuated and/or delayed neutrophil and macrophage infiltration; the expression of vWF, VEGF, and nestin; and the change in vessel volume within muscle in the first 7 d after injury (P < 0.05). By contrast, icing did not influence capillary density in muscle 28 d after injury (P = 0.59). The percentage of immature myofibers relative to the total number of fibers was greater in the icing group than in the sham group 28 d after injury (P = 0.026), but myofiber cross-sectional area did not differ between groups after 7 d (P = 0.35) and 28 d (P = 0.30). In conclusion, although icing disrupted inflammation and some aspects of angiogenesis/revascularization, these effects did not result in substantial differences in capillary density or muscle growth.
Keywords: angiogenesis; cryotherapy; inflammation; muscle injury; regeneration.
Figures
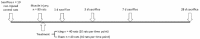





Similar articles
-
Icing after skeletal muscle injury with necrosis in a small fraction of myofibers limits inducible nitric oxide synthase-expressing macrophage invasion and facilitates muscle regeneration.Am J Physiol Regul Integr Comp Physiol. 2023 Apr 1;324(4):R574-R588. doi: 10.1152/ajpregu.00258.2022. Epub 2023 Mar 6. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36878487
-
Icing after eccentric contraction-induced muscle damage perturbs the disappearance of necrotic muscle fibers and phenotypic dynamics of macrophages in mice.J Appl Physiol (1985). 2021 May 1;130(5):1410-1420. doi: 10.1152/japplphysiol.01069.2020. Epub 2021 Mar 25. J Appl Physiol (1985). 2021. PMID: 33764172
-
Icing after skeletal muscle injury decreases M1 macrophage accumulation and TNF-α expression during the early phase of muscle regeneration in rats.Histochem Cell Biol. 2023 Jan;159(1):77-89. doi: 10.1007/s00418-022-02143-8. Epub 2022 Sep 17. Histochem Cell Biol. 2023. PMID: 36114866
-
Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats.J Appl Physiol (1985). 2011 Feb;110(2):382-8. doi: 10.1152/japplphysiol.01187.2010. Epub 2010 Dec 16. J Appl Physiol (1985). 2011. PMID: 21164157
-
The inflammatory response to skeletal muscle injury: illuminating complexities.Sports Med. 2008;38(11):947-69. doi: 10.2165/00007256-200838110-00005. Sports Med. 2008. PMID: 18937524 Review.
Cited by
-
Nestin and osteocrin mRNA increases in human semitendinosus myotendinous junction 7 days after a single bout of eccentric exercise.Histochem Cell Biol. 2022 Jul;158(1):49-64. doi: 10.1007/s00418-022-02101-4. Epub 2022 Apr 15. Histochem Cell Biol. 2022. PMID: 35428952 Clinical Trial.
-
Efficacy of Arm Care Programs for Injury Prevention.Curr Rev Musculoskelet Med. 2021 Apr;14(2):160-167. doi: 10.1007/s12178-021-09694-8. Epub 2021 Jan 22. Curr Rev Musculoskelet Med. 2021. PMID: 33481174 Free PMC article. Review.
-
Controlled Delivery of Corticosteroids Using Tunable Tough Adhesives.Adv Healthc Mater. 2023 Jan;12(3):e2201000. doi: 10.1002/adhm.202201000. Epub 2022 Nov 9. Adv Healthc Mater. 2023. PMID: 36285360 Free PMC article.
-
Modelling the skeletal muscle injury recovery using in vivo contrast-enhanced micro-CT: a proof-of-concept study in a rat model.Eur Radiol Exp. 2020 Jun 3;4(1):33. doi: 10.1186/s41747-020-00163-4. Eur Radiol Exp. 2020. PMID: 32488324 Free PMC article.
-
Local cooling for relieving pain from perineal trauma sustained during childbirth.Cochrane Database Syst Rev. 2020 Oct 9;10(10):CD006304. doi: 10.1002/14651858.CD006304.pub4. Cochrane Database Syst Rev. 2020. PMID: 33034900 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

