A harmine-derived beta-carboline displays anti-cancer effects in vitro by targeting protein synthesis
- PMID: 28322844
- PMCID: PMC5515283
- DOI: 10.1016/j.ejphar.2017.03.034
A harmine-derived beta-carboline displays anti-cancer effects in vitro by targeting protein synthesis
Abstract
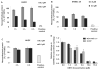
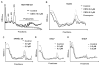
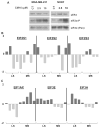
Growing evidence indicates that protein synthesis is deregulated in cancer onset and progression and targeting this process might be a selective way to combat cancers. While harmine is known to inhibit DYRK1A and intercalate into the DNA, tri-substitution was shown previously to modify its activity profile in favor of protein synthesis inhibition. In this study, we thus evaluated the optimized derivative CM16 in vitro anti-cancer effects unfolding its protein synthesis inhibition activity. Indeed, the growth inhibitory profile of CM16 in the NCI 60-cancer-cell-line-panel correlated with those of other compounds described as protein synthesis inhibitors. Accordingly, CM16 decreased in a time- and concentration-dependent manner the translation of neosynthesized proteins in vitro while it did not affect mRNA transcription. CM16 rapidly penetrated into the cell in the perinuclear region of the endoplasmic reticulum where it appears to target translation initiation as highlighted by ribosomal disorganization. More precisely, we found that the mRNA expression levels of the initiation factors EIF1AX, EIF3E and EIF3H differ when comparing resistant or sensitive cell models to CM16. Additionally, CM16 induced eIF2α phosphorylation. Those effects could explain, at least partly, the CM16 cytostatic anti-cancer effects observed in vitro while neither cell cycle arrest nor DNA intercalation could be demonstrated. Therefore, targeting protein synthesis initiation with CM16 could represent a new promising alternative to current cancer therapies due to the specific alterations of the translation machinery in cancer cells as recently evidenced with respect to EIF1AX and eIF3 complex, the potential targets identified in this present study.
Keywords: Beta-carboline; Cancer; Harmine; Protein synthesis; Translation initiation.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
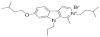
Similar articles
-
Data in support of a harmine-derived beta-carboline in vitro effects in cancer cells through protein synthesis.Data Brief. 2017 May 5;12:546-551. doi: 10.1016/j.dib.2017.05.006. eCollection 2017 Jun. Data Brief. 2017. PMID: 28529967 Free PMC article.
-
A new potential anti-cancer beta-carboline derivative decreases the expression levels of key proteins involved in glioma aggressiveness: A proteomic investigation.Drug Dev Res. 2020 Feb;81(1):32-42. doi: 10.1002/ddr.21600. Epub 2019 Sep 9. Drug Dev Res. 2020. PMID: 31498913
-
Novel trisubstituted harmine derivatives with original in vitro anticancer activity.J Med Chem. 2012 Jul 26;55(14):6489-501. doi: 10.1021/jm300542e. Epub 2012 Jul 18. J Med Chem. 2012. PMID: 22770529
-
Targeting the translation machinery in cancer.Nat Rev Drug Discov. 2015 Apr;14(4):261-78. doi: 10.1038/nrd4505. Epub 2015 Mar 6. Nat Rev Drug Discov. 2015. PMID: 25743081 Review.
-
The biological and therapeutic relevance of mRNA translation in cancer.Nat Rev Clin Oncol. 2011 May;8(5):280-91. doi: 10.1038/nrclinonc.2011.16. Epub 2011 Mar 1. Nat Rev Clin Oncol. 2011. PMID: 21364523 Review.
Cited by
-
Harmine suppresses hyper-activated Ras-MAPK pathway by selectively targeting oncogenic mutated Ras/Raf in Caenorhabditis elegans.Cancer Cell Int. 2019 Jun 11;19:159. doi: 10.1186/s12935-019-0880-4. eCollection 2019. Cancer Cell Int. 2019. PMID: 31198408 Free PMC article.
-
Research progress on the antitumor effects of harmine.Front Oncol. 2024 Mar 25;14:1382142. doi: 10.3389/fonc.2024.1382142. eCollection 2024. Front Oncol. 2024. PMID: 38590646 Free PMC article. Review.
-
Harmine is an effective therapeutic small molecule for the treatment of cardiac hypertrophy.Acta Pharmacol Sin. 2022 Jan;43(1):50-63. doi: 10.1038/s41401-021-00639-y. Epub 2021 Mar 30. Acta Pharmacol Sin. 2022. PMID: 33785860 Free PMC article.
-
Synthetic Biology in Plants, a Boon for Coming Decades.Mol Biotechnol. 2021 Dec;63(12):1138-1154. doi: 10.1007/s12033-021-00386-9. Epub 2021 Aug 21. Mol Biotechnol. 2021. PMID: 34420149 Review.
-
A Quick Guide to Small-Molecule Inhibitors of Eukaryotic Protein Synthesis.Biochemistry (Mosc). 2020 Nov;85(11):1389-1421. doi: 10.1134/S0006297920110097. Biochemistry (Mosc). 2020. PMID: 33280581 Free PMC article. Review.
References
-
- Abbassi R, Johns TG, Kassiou M, Munoz L. DYRK1A in neurodegeneration and cancer: molecular basis and clinical implications. Pharmacol Ther. 2015;151:87–98. - PubMed
-
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. - PubMed
-
- Bannon PG, Dawes J, Dean RT. Malformin A prevents IL-1 induced endothelial changes by inhibition of protein synthesis. Thromb Haemost. 1994;72:482–483. - PubMed
-
- Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14:261–278. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

