Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels
- PMID: 28317216
- PMCID: PMC5413834
- DOI: 10.1002/glia.23138
Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels
Abstract
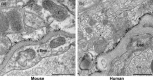
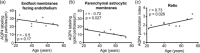
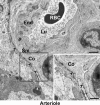
Aquaporin-4 (AQP4), the predominant water channel in the brain, is expressed in astrocytes and ependymal cells. In rodents AQP4 is highly polarized to perivascular astrocytic endfeet and loss of AQP4 polarization is associated with disease. The present study was undertaken to compare the expression pattern of AQP4 in human and mouse cortical astrocytes. Cortical tissue specimens were sampled from 11 individuals undergoing neurosurgery wherein brain tissue was removed as part of the procedure, and compared with cortical tissue from 5 adult wild-type mice processed similarly. The tissue samples were immersion-fixed and prepared for AQP4 immunogold electron microscopy, allowing quantitative assessment of AQP4's subcellular distribution. In mouse we found that AQP4 water channels were prominently clustered around vessels, being 5 to 10-fold more abundant in astrocytic endfoot membranes facing the capillary endothelium than in parenchymal astrocytic membranes. In contrast, AQP4 was markedly less polarized in human astrocytes, being only two to three-fold enriched in astrocytic endfoot membranes adjacent to capillaries. The lower degree of AQP4 polarization in human subjects (1/3 of that in mice) was mainly due to higher AQP4 expression in parenchymal astrocytic membranes. We conclude that there are hitherto unrecognized species differences in AQP4 polarization toward microvessels in the cerebral cortex.
Keywords: AQP4; brain; electron microscopy; endfeet; perivascular.
© 2017 The Authors GLIA Published by Wiley Periodicals, Inc.
Figures




Similar articles
-
Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes.Brain Struct Funct. 2014 Nov;219(6):2181-6. doi: 10.1007/s00429-013-0629-0. Epub 2013 Aug 28. Brain Struct Funct. 2014. PMID: 23982198 Free PMC article.
-
Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus.Glia. 2019 Jan;67(1):91-100. doi: 10.1002/glia.23528. Epub 2018 Oct 10. Glia. 2019. PMID: 30306658
-
Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer's disease.J Alzheimers Dis. 2011;27(4):711-22. doi: 10.3233/JAD-2011-110725. J Alzheimers Dis. 2011. PMID: 21891870
-
Aquaporin-4 in Astroglial Cells in the CNS and Supporting Cells of Sensory Organs-A Comparative Perspective.Int J Mol Sci. 2016 Aug 26;17(9):1411. doi: 10.3390/ijms17091411. Int J Mol Sci. 2016. PMID: 27571065 Free PMC article. Review.
-
Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1.Neuroscience. 2004;129(4):905-13. doi: 10.1016/j.neuroscience.2004.08.053. Neuroscience. 2004. PMID: 15561407 Review.
Cited by
-
The Water Transport System in Astrocytes-Aquaporins.Cells. 2022 Aug 18;11(16):2564. doi: 10.3390/cells11162564. Cells. 2022. PMID: 36010640 Free PMC article. Review.
-
The Brain's Waste-Removal System.Cerebrum. 2018 Jul 1;2018:cer-09-18. eCollection 2018 Jul-Aug. Cerebrum. 2018. PMID: 30746031 Free PMC article.
-
Waste Clearance in the Brain.Front Neuroanat. 2021 Jul 7;15:665803. doi: 10.3389/fnana.2021.665803. eCollection 2021. Front Neuroanat. 2021. PMID: 34305538 Free PMC article. Review.
-
Edaravone Exerts Brain Protective Function by Reducing the Expression of AQP4, APP and Aβ Proteins.Open Life Sci. 2019 Dec 31;14:651-658. doi: 10.1515/biol-2019-0074. eCollection 2019 Jan. Open Life Sci. 2019. PMID: 33817204 Free PMC article.
-
Current Understanding of the Anatomy, Physiology, and Magnetic Resonance Imaging of Neurofluids: Update From the 2022 "ISMRM Imaging Neurofluids Study group" Workshop in Rome.J Magn Reson Imaging. 2024 Feb;59(2):431-449. doi: 10.1002/jmri.28759. Epub 2023 May 4. J Magn Reson Imaging. 2024. PMID: 37141288 Free PMC article. Review.
References
-
- Alvestad, S. , Hammer, J. , Hoddevik, E. H. , Skare, O. , Sonnewald, U. , Amiry‐Moghaddam, M. , et al. (2013). Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Research, 105, 30–41. - PubMed
-
- Amiry‐Moghaddam, M. , Otsuka, T. , Hurn, P. D. , Traystman, R. J. , Haug, F. M. , Froehner, S. C. , et al. (2003). An alpha‐syntrophin‐dependent pool of AQP4 in astroglial end‐feet confers bidirectional water flow between blood and brain. Proceedings of the National Academy of Science of United States of America, 100, 2106–2111. - PMC - PubMed
-
- Amiry‐Moghaddam, M. & Ottersen, O. P. (2013). Immunogold cytochemistry in neuroscience. Nature Neuroscience, 16, 798–804. - PubMed
-
- Amiry‐Moghaddam, M. , Xue, R. , Haug, F. M. , Neely, J. D. , Bhardwaj, A. , Agre, P. , et al. (2004). Alpha‐syntrophin deletion removes the perivascular but not endothelial pool of aquaporin‐4 at the blood‐brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB Journal, 18, 542–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

