Insights into the molecular architecture and histone H3-H4 deposition mechanism of yeast Chromatin assembly factor 1
- PMID: 28315525
- PMCID: PMC5404918
- DOI: 10.7554/eLife.23474
Insights into the molecular architecture and histone H3-H4 deposition mechanism of yeast Chromatin assembly factor 1
Abstract
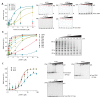
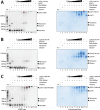
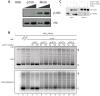
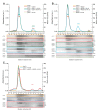
How the very first step in nucleosome assembly, deposition of histone H3-H4 as tetramers or dimers on DNA, is accomplished remains largely unclear. Here, we report that yeast chromatin assembly factor 1 (CAF1), a conserved histone chaperone complex that deposits H3-H4 during DNA replication, binds a single H3-H4 heterodimer in solution. We identify a new DNA-binding domain in the large Cac1 subunit of CAF1, which is required for high-affinity DNA binding by the CAF1 three-subunit complex, and which is distinct from the previously described C-terminal winged-helix domain. CAF1 binds preferentially to DNA molecules longer than 40 bp, and two CAF1-H3-H4 complexes concertedly associate with DNA molecules of this size, resulting in deposition of H3-H4 tetramers. While DNA binding is not essential for H3-H4 tetrasome deposition in vitro, it is required for efficient DNA synthesis-coupled nucleosome assembly. Mutant histones with impaired H3-H4 tetramerization interactions fail to release from CAF1, indicating that DNA deposition of H3-H4 tetramers by CAF1 requires a hierarchical cooperation between DNA binding, H3-H4 deposition and histone tetramerization.
Keywords: DNA replication; S. cerevisiae; biochemistry; biophysics; chromatin; chromatin assembly factor 1; histone chaperone; histones; structural biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures







Similar articles
-
DNA-mediated association of two histone-bound complexes of yeast Chromatin Assembly Factor-1 (CAF-1) drives tetrasome assembly in the wake of DNA replication.Elife. 2017 Mar 18;6:e22799. doi: 10.7554/eLife.22799. Elife. 2017. PMID: 28315523 Free PMC article.
-
The Cac1 subunit of histone chaperone CAF-1 organizes CAF-1-H3/H4 architecture and tetramerizes histones.Elife. 2016 Sep 30;5:e18023. doi: 10.7554/eLife.18023. Elife. 2016. PMID: 27690308 Free PMC article.
-
Splitting of H3-H4 tetramers at transcriptionally active genes undergoing dynamic histone exchange.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1296-301. doi: 10.1073/pnas.1018308108. Epub 2011 Jan 10. Proc Natl Acad Sci U S A. 2011. PMID: 21220302 Free PMC article.
-
Mechanistic insights into histone deposition and nucleosome assembly by the chromatin assembly factor-1.Nucleic Acids Res. 2018 Nov 2;46(19):9907-9917. doi: 10.1093/nar/gky823. Nucleic Acids Res. 2018. PMID: 30239791 Free PMC article. Review.
-
All roads lead to chromatin: multiple pathways for histone deposition.Biochim Biophys Acta. 2013 Mar-Apr;1819(3-4):238-46. Biochim Biophys Acta. 2013. PMID: 24459726 Review.
Cited by
-
Variation, Variegation and Heritable Gene Repression in S. cerevisiae.Front Genet. 2021 Mar 4;12:630506. doi: 10.3389/fgene.2021.630506. eCollection 2021. Front Genet. 2021. PMID: 33747046 Free PMC article. Review.
-
Molecular basis for chromatin assembly and modification by multiprotein complexes.Protein Sci. 2019 Feb;28(2):329-343. doi: 10.1002/pro.3535. Epub 2018 Dec 13. Protein Sci. 2019. PMID: 30350439 Free PMC article. Review.
-
The histone chaperone CAF-1 cooperates with the DNA methyltransferases to maintain Cd4 silencing in cytotoxic T cells.Genes Dev. 2019 Jun 1;33(11-12):669-683. doi: 10.1101/gad.322024.118. Epub 2019 Apr 11. Genes Dev. 2019. PMID: 30975723 Free PMC article.
-
Lagging strand gap suppression connects BRCA-mediated fork protection to nucleosome assembly through PCNA-dependent CAF-1 recycling.Nat Commun. 2022 Sep 9;13(1):5323. doi: 10.1038/s41467-022-33028-y. Nat Commun. 2022. PMID: 36085347 Free PMC article.
-
Structure of the Hir histone chaperone complex.Mol Cell. 2024 Jul 25;84(14):2601-2617.e12. doi: 10.1016/j.molcel.2024.05.031. Epub 2024 Jun 25. Mol Cell. 2024. PMID: 38925115
References
-
- Abascal F, Corpet A, Gurard-Levin ZA, Juan D, Ochsenbein F, Rico D, Valencia A, Almouzni G. Subfunctionalization via adaptive evolution influenced by genomic context: the case of histone chaperones ASF1a and ASF1b. Molecular Biology and Evolution. 2013;30:1853–1866. doi: 10.1093/molbev/mst086. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

