Astrocyte Ca2+ Influx Negatively Regulates Neuronal Activity
- PMID: 28303263
- PMCID: PMC5348542
- DOI: 10.1523/ENEURO.0340-16.2017
Astrocyte Ca2+ Influx Negatively Regulates Neuronal Activity
Abstract
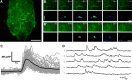
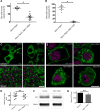
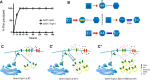
Maintenance of neural circuit activity requires appropriate regulation of excitatory and inhibitory synaptic transmission. Recently, glia have emerged as key partners in the modulation of neuronal excitability; however, the mechanisms by which glia regulate neuronal signaling are still being elucidated. Here, we describe an analysis of how Ca2+ signals within Drosophila astrocyte-like glia regulate excitability in the nervous system. We find that Drosophila astrocytes exhibit robust Ca2+ oscillatory activity manifested by fast, recurrent microdomain Ca2+ fluctuations within processes that infiltrate the synaptic neuropil. Unlike the enhanced neuronal activity and behavioral seizures that were previously observed during manipulations that trigger Ca2+ influx into Drosophila cortex glia, we find that acute induction of astrocyte Ca2+ influx leads to a rapid onset of behavioral paralysis and a suppression of neuronal activity. We observe that Ca2+ influx triggers rapid endocytosis of the GABA transporter (GAT) from astrocyte plasma membranes, suggesting that increased synaptic GABA levels contribute to the neuronal silencing and paralysis. We identify Rab11 as a novel regulator of GAT trafficking that is required for this form of activity regulation. Suppression of Rab11 function strongly offsets the reduction of neuronal activity caused by acute astrocyte Ca2+ influx, likely by inhibiting GAT endocytosis. Our data provide new insights into astrocyte Ca2+ signaling and indicate that distinct glial subtypes in the Drosophila brain can mediate opposing effects on neuronal excitability.
Keywords: Ca2+; Drosophila; GABA; GAT; Rab11; astrocyte.
Figures


Similar articles
-
TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3.Nat Neurosci. 2011 Dec 11;15(1):70-80. doi: 10.1038/nn.3000. Nat Neurosci. 2011. PMID: 22158513 Free PMC article.
-
Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus.Glia. 2016 Dec;64(12):2093-2103. doi: 10.1002/glia.23042. Epub 2016 Aug 1. Glia. 2016. PMID: 27479868
-
Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes.J Biol Chem. 2011 Nov 25;286(47):40464-76. doi: 10.1074/jbc.M111.232009. Epub 2011 Oct 3. J Biol Chem. 2011. PMID: 21969376 Free PMC article.
-
Local energy on demand: Are 'spontaneous' astrocytic Ca2+-microdomains the regulatory unit for astrocyte-neuron metabolic cooperation?Brain Res Bull. 2018 Jan;136:54-64. doi: 10.1016/j.brainresbull.2017.04.011. Epub 2017 Apr 24. Brain Res Bull. 2018. PMID: 28450076 Review.
-
Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology.Biomolecules. 2021 Oct 6;11(10):1467. doi: 10.3390/biom11101467. Biomolecules. 2021. PMID: 34680100 Free PMC article. Review.
Cited by
-
Function and therapeutic value of astrocytes in neurological diseases.Nat Rev Drug Discov. 2022 May;21(5):339-358. doi: 10.1038/s41573-022-00390-x. Epub 2022 Feb 16. Nat Rev Drug Discov. 2022. PMID: 35173313 Free PMC article. Review.
-
Endocytosis at the Drosophila blood-brain barrier as a function for sleep.Elife. 2018 Nov 26;7:e43326. doi: 10.7554/eLife.43326. Elife. 2018. PMID: 30475209 Free PMC article.
-
Astrocytic Calcium Dynamics Along the Pain Pathway.Front Cell Neurosci. 2020 Oct 16;14:594216. doi: 10.3389/fncel.2020.594216. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192331 Free PMC article. Review.
-
TrpML-mediated astrocyte microdomain Ca2+ transients regulate astrocyte-tracheal interactions.Elife. 2020 Dec 7;9:e58952. doi: 10.7554/eLife.58952. Elife. 2020. PMID: 33284108 Free PMC article.
-
Glial Tiling in the Insect Nervous System.Front Cell Neurosci. 2022 Feb 17;16:825695. doi: 10.3389/fncel.2022.825695. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35250488 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
