A Potential Role of Salmonella Infection in the Onset of Inflammatory Bowel Diseases
- PMID: 28293241
- PMCID: PMC5329042
- DOI: 10.3389/fimmu.2017.00191
A Potential Role of Salmonella Infection in the Onset of Inflammatory Bowel Diseases
Abstract
Inflammatory bowel disease (IBD) includes a set of pathologies that result from a deregulated immune response that may affect any portion of the gastrointestinal tract. The most prevalent and defined forms of IBD are Crohn's disease and ulcerative colitis. Although the etiology of IBD is not well defined, it has been suggested that environmental and genetic factors contribute to disease development and that the interaction between these two factors can trigger the pathology. Diet, medication use, vitamin D status, smoking, and bacterial infections have been proposed to influence or contribute to the onset or development of the disease in susceptible individuals. The infection with pathogenic bacteria is a key factor that can influence the development and severity of this disease. Here, we present a comprehensive review of studies performed in human and mice susceptible to IBD, which supports the notion that infection with bacterial pathogens, such as Salmonella, could promote the onset of IBD due to permanent changes in the intestinal microbiota, disruption of the epithelial barrier and alterations of the intestinal immune response after infection.
Keywords: Crohn’s disease; Salmonella enterica serovar Typhimurium; gut microbiota; inflammatory bowel disease; innate immune response; ulcerative colitis; virulence factors.
Figures
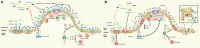
Similar articles
-
Dismicrobism in inflammatory bowel disease and colorectal cancer: changes in response of colocytes.World J Gastroenterol. 2014 Dec 28;20(48):18121-30. doi: 10.3748/wjg.v20.i48.18121. World J Gastroenterol. 2014. PMID: 25561781 Free PMC article. Review.
-
Contentious host-microbiota relationship in inflammatory bowel disease--can foes become friends again?Scand J Gastroenterol. 2015 Jan;50(1):34-42. doi: 10.3109/00365521.2014.966320. Scand J Gastroenterol. 2015. PMID: 25523554 Review.
-
The Intestinal Microbiota in Inflammatory Bowel Disease.ILAR J. 2015;56(2):192-204. doi: 10.1093/ilar/ilv030. ILAR J. 2015. PMID: 26323629 Review.
-
Intestinal microbiota and ulcerative colitis.J Infect Chemother. 2015 Nov;21(11):761-8. doi: 10.1016/j.jiac.2015.07.010. Epub 2015 Sep 4. J Infect Chemother. 2015. PMID: 26346678 Review.
-
Metabolome and inflammasome in inflammatory bowel disease.Transl Res. 2012 Jul;160(1):1-28. doi: 10.1016/j.trsl.2011.08.006. Epub 2011 Sep 14. Transl Res. 2012. PMID: 22687960 Review.
Cited by
-
Implications of Gut Microbiota in Complex Human Diseases.Int J Mol Sci. 2021 Nov 23;22(23):12661. doi: 10.3390/ijms222312661. Int J Mol Sci. 2021. PMID: 34884466 Free PMC article. Review.
-
Non-Hematopoietic MLKL Protects Against Salmonella Mucosal Infection by Enhancing Inflammasome Activation.Front Immunol. 2018 Feb 2;9:119. doi: 10.3389/fimmu.2018.00119. eCollection 2018. Front Immunol. 2018. PMID: 29456533 Free PMC article.
-
Complex probiotics alleviate ampicillin-induced antibiotic-associated diarrhea in mice.Front Microbiol. 2023 Apr 14;14:1156058. doi: 10.3389/fmicb.2023.1156058. eCollection 2023. Front Microbiol. 2023. PMID: 37125182 Free PMC article.
-
The Preventive Effect of Lactobacillus plantarum ZS62 on DSS-Induced IBD by Regulating Oxidative Stress and the Immune Response.Oxid Med Cell Longev. 2021 Oct 27;2021:9416794. doi: 10.1155/2021/9416794. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34745426 Free PMC article.
-
Role of intestinal microbiota and metabolites in inflammatory bowel disease.Chin Med J (Engl). 2019 Jul 5;132(13):1610-1614. doi: 10.1097/CM9.0000000000000290. Chin Med J (Engl). 2019. PMID: 31090547 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

