Celiac Disease: Role of the Epithelial Barrier
- PMID: 28275682
- PMCID: PMC5331784
- DOI: 10.1016/j.jcmgh.2016.12.006
Celiac Disease: Role of the Epithelial Barrier
Abstract
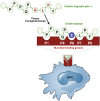
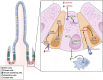
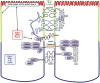
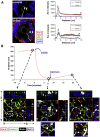
In celiac disease (CD) a T-cell-mediated response to gluten is mounted in genetically predisposed individuals, resulting in a malabsorptive enteropathy histologically highlighted by villous atrophy and crypt hyperplasia. Recent data point to the epithelial layer as an under-rated hot spot in celiac pathophysiology to date. This overview summarizes current functional and genetic evidence on the role of the epithelial barrier in CD, consisting of the cell membranes and the apical junctional complex comprising sealing as well as ion and water channel-forming tight junction proteins and the adherens junction. Moreover, the underlying mechanisms are discussed, including apoptosis of intestinal epithelial cells, biology of intestinal stem cells, alterations in the apical junctional complex, transcytotic uptake of gluten peptides, and possible implications of a defective epithelial polarity. Current research is directed toward new treatment options for CD that are alternatives or complementary therapeutics to a gluten-free diet. Thus, strategies to target an altered epithelial barrier therapeutically also are discussed.
Keywords: Bmp, bone morphogenetic protein; CBC, crypt base columnar cell; CD, celiac disease; Celiac Sprue; EGF, epidermal growth factor; Epithelial Polarity; GFD, gluten-free diet; GI, gastrointestinal; GWAS, genome-wide association studies; Gluten-Sensitive Enteropathy; IEC, intestinal epithelial cell; IL, interleukin; MIC-A, major histocompatibility complex class I chain–related gene-A; Partitioning-Defective Proteins; SNP, single-nucleotide polymorphism; TJ, tight junction; Tight Junction; ZO, zonula occludens; aPKC, atypical protein kinase C; α-Gliadin 33mer.
Figures
Similar articles
-
Monitoring nonresponsive patients who have celiac disease.Gastrointest Endosc Clin N Am. 2006 Apr;16(2):317-27. doi: 10.1016/j.giec.2006.03.005. Gastrointest Endosc Clin N Am. 2006. PMID: 16644460 Review.
-
Celiac disease: risk assessment, diagnosis, and monitoring.Mol Diagn Ther. 2008;12(5):289-98. doi: 10.1007/BF03256294. Mol Diagn Ther. 2008. PMID: 18803427 Review.
-
Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue.Gut. 2008 Jun;57(6):747-54. doi: 10.1136/gut.2007.136366. Epub 2008 Feb 27. Gut. 2008. PMID: 18305066
-
Mechanisms of Intestinal Epithelial Barrier Dysfunction by Adherent-Invasive Escherichia coli.Cell Mol Gastroenterol Hepatol. 2016 Oct 22;3(1):41-50. doi: 10.1016/j.jcmgh.2016.10.004. eCollection 2017 Jan. Cell Mol Gastroenterol Hepatol. 2016. PMID: 28174756 Free PMC article. Review.
-
Celiac disease: from gluten to autoimmunity.Autoimmun Rev. 2008 Sep;7(8):644-50. doi: 10.1016/j.autrev.2008.05.006. Epub 2008 Jun 25. Autoimmun Rev. 2008. PMID: 18589004 Review.
Cited by
-
Human gut derived-organoids provide model to study gluten response and effects of microbiota-derived molecules in celiac disease.Sci Rep. 2019 May 7;9(1):7029. doi: 10.1038/s41598-019-43426-w. Sci Rep. 2019. PMID: 31065051 Free PMC article.
-
Celiac Disease Is Associated with Microscopic Colitis in Refractory Cases in Adults: A Systematic Review and Meta-Analysis of Observational Studies.Dig Dis Sci. 2022 Aug;67(8):3529-3542. doi: 10.1007/s10620-021-07232-7. Epub 2021 Aug 27. Dig Dis Sci. 2022. PMID: 34448981 Review.
-
Celiac Disease Defined by Over-Sensitivity to Gliadin Activation and Superior Antigen Presentation of Dendritic Cells.Int J Mol Sci. 2021 Sep 15;22(18):9982. doi: 10.3390/ijms22189982. Int J Mol Sci. 2021. PMID: 34576145 Free PMC article.
-
Study of Environmental Enteropathy and Malnutrition (SEEM) in Pakistan: protocols for biopsy based biomarker discovery and validation.BMC Pediatr. 2019 Jul 22;19(1):247. doi: 10.1186/s12887-019-1564-x. BMC Pediatr. 2019. PMID: 31331393 Free PMC article.
-
A Randomized, Placebo-Controlled, Pilot Clinical Trial to Evaluate the Effect of Supplementation with Prebiotic Synergy 1 on Iron Homeostasis in Children and Adolescents with Celiac Disease Treated with a Gluten-Free Diet.Nutrients. 2018 Nov 21;10(11):1818. doi: 10.3390/nu10111818. Nutrients. 2018. PMID: 30469412 Free PMC article. Clinical Trial.
References
-
- Felber J., Aust D., Baas S. Ergebnisse einer S2k-Konsensuskonferenz der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselerkrankungen (DGVS) gemeinsam mit der Deutschen Zöliakie-Gesellschaft (DZG e. V.) zur Zöliakie, Weizenallergie und Weizensensitivität. Z Gastroenterol. 2014;52:711–743. - PubMed
-
- Fasano A., Berti I., Gerarduzzi T. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. - PubMed
-
- Lewis N.R., Holmes G.K. Risk of morbidity in contemporary celiac disease. Expert Rev Gastroenterol Hepatol. 2010;4:767–780. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

