Quantitative Evaluation of Protein Heterogeneity within Herpes Simplex Virus 1 Particles
- PMID: 28275191
- PMCID: PMC5411592
- DOI: 10.1128/JVI.00320-17
Quantitative Evaluation of Protein Heterogeneity within Herpes Simplex Virus 1 Particles
Abstract
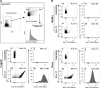
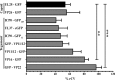
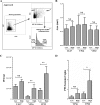
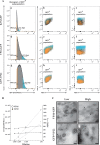
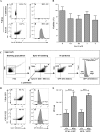
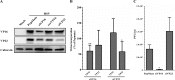
Several virulence genes have been identified thus far in the herpes simplex virus 1 genome. It is also generally accepted that protein heterogeneity among virions further impacts viral fitness. However, linking this variability directly with infectivity has been challenging at the individual viral particle level. To address this issue, we resorted to flow cytometry (flow virometry), a powerful approach we recently employed to analyze individual viral particles, to identify which tegument proteins vary and directly address if such variability is biologically relevant. We found that the stoichiometry of the UL37, ICP0, and VP11/12 tegument proteins in virions is more stable than the VP16 and VP22 tegument proteins, which varied significantly among viral particles. Most interestingly, viruses sorted for their high VP16 or VP22 content yielded modest but reproducible increases in infectivity compared to their corresponding counterparts containing low VP16 or VP22 content. These findings were corroborated for VP16 in short interfering RNA experiments but proved intriguingly more complex for VP22. An analysis by quantitative Western blotting revealed substantial alterations of virion composition upon manipulation of individual tegument proteins and suggests that VP22 protein levels acted indirectly on viral fitness. These findings reaffirm the interdependence of the virion components and corroborate that viral fitness is influenced not only by the genome of viruses but also by the stoichiometry of proteins within each virion.IMPORTANCE The ability of viruses to spread in animals has been mapped to several viral genes, but other factors are clearly involved, including virion heterogeneity. To directly probe whether the latter influences viral fitness, we analyzed the protein content of individual herpes simplex virus 1 particles using an innovative flow cytometry approach. The data confirm that some viral proteins are incorporated in more controlled amounts, while others vary substantially. Interestingly, this correlates with the VP16 trans-activating viral protein and indirectly with VP22, a second virion component whose modulation profoundly alters virion composition. This reaffirms that not only the presence but also the amount of specific tegument proteins is an important determinant of viral fitness.
Keywords: FACS; HSV-1; VP16; VP22; flow cytometry; flow virometry; herpes simplex virus; heterogeneity; tegument; viral particle.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Incorporation of the herpes simplex virus type 1 tegument protein VP22 into the virus particle is independent of interaction with VP16.Virology. 2007 Dec 20;369(2):263-80. doi: 10.1016/j.virol.2007.07.020. Epub 2007 Sep 20. Virology. 2007. PMID: 17888478
-
VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells.J Virol. 1995 Dec;69(12):7932-41. doi: 10.1128/JVI.69.12.7932-7941.1995. J Virol. 1995. PMID: 7494306 Free PMC article.
-
Assembly of infectious Herpes simplex virus type 1 virions in the absence of full-length VP22.J Virol. 2000 Nov;74(21):10041-54. doi: 10.1128/jvi.74.21.10041-10054.2000. J Virol. 2000. PMID: 11024133 Free PMC article.
-
[Research Advances in VP16 of the Herpes Virus].Bing Du Xue Bao. 2016 Nov;32(6):817-24. Bing Du Xue Bao. 2016. PMID: 30004657 Review. Chinese.
-
Early expression of herpes simplex virus (HSV) proteins and reactivation of latent infection.Folia Microbiol (Praha). 2000;45(1):7-28. doi: 10.1007/BF02817445. Folia Microbiol (Praha). 2000. PMID: 11200675 Review.
Cited by
-
Flow Virometry: a Powerful Tool To Functionally Characterize Viruses.J Virol. 2018 Jan 17;92(3):e01765-17. doi: 10.1128/JVI.01765-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29167334 Free PMC article. Review.
-
Analysis of a fully infectious bio-orthogonally modified human virus reveals novel features of virus cell entry.PLoS Pathog. 2019 Oct 7;15(10):e1007956. doi: 10.1371/journal.ppat.1007956. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31589653 Free PMC article.
-
Flow virometry for process monitoring of live virus vaccines-lessons learned from ERVEBO.Sci Rep. 2021 Apr 1;11(1):7432. doi: 10.1038/s41598-021-86688-z. Sci Rep. 2021. PMID: 33795759 Free PMC article.
-
Applying Flow Virometry to Study the HIV Envelope Glycoprotein and Differences Across HIV Model Systems.Viruses. 2024 Jun 9;16(6):935. doi: 10.3390/v16060935. Viruses. 2024. PMID: 38932227 Free PMC article.
-
Flow virometry as a tool to study viruses.Methods. 2018 Feb 1;134-135:87-97. doi: 10.1016/j.ymeth.2017.12.011. Epub 2017 Dec 16. Methods. 2018. PMID: 29258922 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

