Discoidin domain receptor 2 mediates collagen-induced activation of membrane-type 1 matrix metalloproteinase in human fibroblasts
- PMID: 28270508
- PMCID: PMC5399112
- DOI: 10.1074/jbc.M116.770057
Discoidin domain receptor 2 mediates collagen-induced activation of membrane-type 1 matrix metalloproteinase in human fibroblasts
Abstract
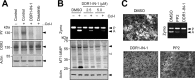
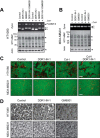
Membrane-type 1 matrix metalloproteinase (MT1-MMP) is a membrane-bound MMP that is highly expressed in cells with invading capacity, including fibroblasts and invasive cancer cells. However, pathways of MT1-MMP up-regulation are not clearly understood. A potential physiological stimulus for MT1-MMP expression is fibrillar collagen, and it has been shown that it up-regulates both MT1-MMP gene and functions in various cell types. However, the mechanisms of collagen-mediated MT1-MMP activation and its physiological relevance are not known. In this study, we identified discoidin domain receptor 2 (DDR2) as a crucial receptor that mediates this process in human fibroblasts. Knocking down DDR2, but not the β1 integrin subunit, a common subunit for all collagen-binding integrins, inhibited the collagen-induced MT1-MMP-dependent activation of pro-MMP-2 and up-regulation of MT1-MMP at the gene and protein levels. Interestingly, DDR2 knockdown or pharmacological inhibition of DDR2 also inhibited the MT1-MMP-dependent cellular degradation of collagen film, suggesting that cell-surface collagen degradation by MT1-MMP involves DDR2-mediated collagen signaling. This DDR2-mediated mechanism is only present in non-transformed mesenchymal cells as collagen-induced MT1-MMP activation in HT1080 fibrosarcoma cells and MT1-MMP function in MDA-MB231 breast cancer cells were not affected by DDR kinase inhibition. DDR2 activation was found to be noticeably more effective when cells were stimulated by collagen without the non-helical telopeptide region compared with intact collagen fibrils. Furthermore, DDR2-dependent MT1-MMP activation by cartilage was found to be more efficient when the tissue was partially damaged. These data suggest that DDR2 is a microenvironment sensor that regulates fibroblast migration in a collagen-rich environment.
Keywords: DDR2; MMP-2; MT1-MMP; arthritis; cancer; collagen; fibroblast; matrix metalloproteinase (MMP).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
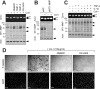
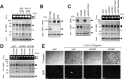
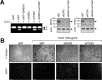

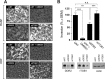

Similar articles
-
Active MT1-MMP is tethered to collagen fibers in DDR2-containing remnants.Gene. 2021 Jul 1;788:145673. doi: 10.1016/j.gene.2021.145673. Epub 2021 Apr 18. Gene. 2021. PMID: 33882324
-
Discoidin Domain Receptor 2 Signaling Regulates Fibroblast Apoptosis through PDK1/Akt.Am J Respir Cell Mol Biol. 2018 Sep;59(3):295-305. doi: 10.1165/rcmb.2017-0419OC. Am J Respir Cell Mol Biol. 2018. PMID: 29652518 Free PMC article.
-
Discoidin Domain Receptor 2 Regulates AT1R Expression in Angiotensin II-Stimulated Cardiac Fibroblasts via Fibronectin-Dependent Integrin-β1 Signaling.Int J Mol Sci. 2021 Aug 28;22(17):9343. doi: 10.3390/ijms22179343. Int J Mol Sci. 2021. PMID: 34502259 Free PMC article.
-
Phagocytosis of collagen by fibroblasts and invasive cancer cells is mediated by MT1-MMP.Biochem Soc Trans. 2007 Aug;35(Pt 4):704-6. doi: 10.1042/BST0350704. Biochem Soc Trans. 2007. PMID: 17635128 Review.
-
Discoidin domain receptor 2: An emerging pharmacological drug target for prospective therapy against osteoarthritis.Pharmacol Rep. 2019 Jun;71(3):399-408. doi: 10.1016/j.pharep.2019.01.007. Epub 2019 Jan 15. Pharmacol Rep. 2019. PMID: 31003149 Review.
Cited by
-
Discoidin Domain Receptors, DDR1b and DDR2, Promote Tumour Growth within Collagen but DDR1b Suppresses Experimental Lung Metastasis in HT1080 Xenografts.Sci Rep. 2020 Feb 11;10(1):2309. doi: 10.1038/s41598-020-59028-w. Sci Rep. 2020. PMID: 32047176 Free PMC article.
-
Recombinant collagen for the repair of skin wounds and photo-aging damage.Regen Biomater. 2024 Sep 2;11:rbae108. doi: 10.1093/rb/rbae108. eCollection 2024. Regen Biomater. 2024. PMID: 39323745 Free PMC article.
-
The biological effect of recombinant humanized collagen on damaged skin induced by UV-photoaging: An in vivo study.Bioact Mater. 2021 Oct 22;11:154-165. doi: 10.1016/j.bioactmat.2021.10.004. eCollection 2022 May. Bioact Mater. 2021. PMID: 34938920 Free PMC article.
-
Discoidin domain receptors: Microenvironment sensors that promote cellular migration and invasion.Cell Adh Migr. 2018;12(4):378-385. doi: 10.1080/19336918.2018.1460011. Epub 2018 May 8. Cell Adh Migr. 2018. PMID: 29671358 Free PMC article. Review.
-
The Charming World of the Extracellular Matrix: A Dynamic and Protective Network of the Intestinal Wall.Front Med (Lausanne). 2021 Apr 16;8:610189. doi: 10.3389/fmed.2021.610189. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33937276 Free PMC article. Review.
References
-
- Itoh Y. (2015) Membrane-type matrix metalloproteinases: their functions and regulations. Matrix Biol. 44-46, 207–223 - PubMed
-
- Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S., Allen E., Chung D., and Weiss S. J. (2004) Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 167, 769–781 - PMC - PubMed
-
- Overall C. M., and Sodek J. (1990) Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. Induction and endogenous activation of collagenase, 72-kDa gelatinase, and Pump-1 is accompanied by the suppression of the tissue inhibitor of matrix metalloproteinases. J. Biol. Chem. 265, 21141–21151 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

