Targeting mTOR Signaling Can Prevent the Progression of FSGS
- PMID: 28270414
- PMCID: PMC5491276
- DOI: 10.1681/ASN.2016050519
Targeting mTOR Signaling Can Prevent the Progression of FSGS
Abstract
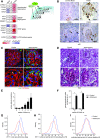
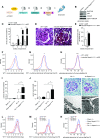
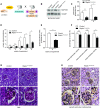
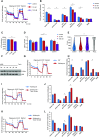
Mammalian target of rapamycin (mTOR) signaling is involved in a variety of kidney diseases. Clinical trials administering mTOR inhibitors to patients with FSGS, a prototypic podocyte disease, led to conflicting results, ranging from remission to deterioration of kidney function. Here, we combined complex genetic titration of mTOR complex 1 (mTORC1) levels in murine glomerular disease models, pharmacologic studies, and human studies to precisely delineate the role of mTOR in FSGS. mTORC1 target genes were significantly induced in microdissected glomeruli from both patients with FSGS and a murine FSGS model. Furthermore, a mouse model with constitutive mTORC1 activation closely recapitulated human FSGS. Notably, the complete knockout of mTORC1 by induced deletion of both Raptor alleles accelerated the progression of murine FSGS models. However, lowering mTORC1 signaling by deleting just one Raptor allele ameliorated the progression of glomerulosclerosis. Similarly, low-dose treatment with the mTORC1 inhibitor rapamycin efficiently diminished disease progression. Mechanistically, complete pharmacologic inhibition of mTOR in immortalized podocytes shifted the cellular energy metabolism toward reduced rates of oxidative phosphorylation and anaerobic glycolysis, which correlated with increased production of reactive oxygen species. Together, these data suggest that podocyte injury and loss is commonly followed by adaptive mTOR activation. Prolonged mTOR activation, however, results in a metabolic podocyte reprogramming leading to increased cellular stress and dedifferentiation, thus offering a treatment rationale for incomplete mTOR inhibition.
Keywords: Pathophysiology of Renal Disease and Progression; focal segmental glomerulosclerosis; mTOR; mitochondrial function; rapamycin; raptor.
Copyright © 2017 by the American Society of Nephrology.
Figures
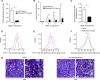
Comment in
-
Glomerular disease: mTOR in FSGS.Nat Rev Nephrol. 2017 May;13(5):260. doi: 10.1038/nrneph.2017.42. Epub 2017 Mar 27. Nat Rev Nephrol. 2017. PMID: 28344329 No abstract available.
Similar articles
-
Role of mTOR in podocyte function and diabetic nephropathy in humans and mice.J Clin Invest. 2011 Jun;121(6):2197-209. doi: 10.1172/JCI44774. Epub 2011 May 23. J Clin Invest. 2011. PMID: 21606591 Free PMC article.
-
Inhibition of mTOR delayed but could not prevent experimental collapsing focal segmental glomerulosclerosis.Sci Rep. 2020 May 22;10(1):8580. doi: 10.1038/s41598-020-65352-y. Sci Rep. 2020. PMID: 32444668 Free PMC article.
-
Mammalian target of rapamycin and tuberous sclerosis complex.J Dermatol Sci. 2015 Aug;79(2):93-100. doi: 10.1016/j.jdermsci.2015.04.005. Epub 2015 Apr 25. J Dermatol Sci. 2015. PMID: 26051878 Review.
-
Loss of mTOR signaling affects cone function, cone structure and expression of cone specific proteins without affecting cone survival.Exp Eye Res. 2015 Jun;135:1-13. doi: 10.1016/j.exer.2015.04.006. Epub 2015 Apr 14. Exp Eye Res. 2015. PMID: 25887293 Free PMC article.
-
The role of mechanistic target of rapamycin in maintenance of glomerular epithelial cells.Curr Opin Nephrol Hypertens. 2016 Jan;25(1):28-34. doi: 10.1097/MNH.0000000000000181. Curr Opin Nephrol Hypertens. 2016. PMID: 26625863 Free PMC article. Review.
Cited by
-
High-throughput data on circular RNA reveal novel insights into chronic glomerulonephritis.Genes Genomics. 2023 Apr;45(4):475-490. doi: 10.1007/s13258-022-01320-2. Epub 2022 Oct 20. Genes Genomics. 2023. PMID: 36264417
-
Emodin improves renal fibrosis in chronic kidney disease by regulating mitochondrial homeostasis through the mediation of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α).Eur J Histochem. 2024 May 13;68(2):3917. doi: 10.4081/ejh.2024.3917. Eur J Histochem. 2024. PMID: 38742403 Free PMC article.
-
Deconvolution of Focal Segmental Glomerulosclerosis Pathophysiology Using Transcriptomics Techniques.Glomerular Dis. 2021 Jul 14;1(4):265-276. doi: 10.1159/000518404. eCollection 2021 Oct. Glomerular Dis. 2021. PMID: 36751384 Free PMC article. Review.
-
Anaerobic Glycolysis Maintains the Glomerular Filtration Barrier Independent of Mitochondrial Metabolism and Dynamics.Cell Rep. 2019 Apr 30;27(5):1551-1566.e5. doi: 10.1016/j.celrep.2019.04.012. Cell Rep. 2019. PMID: 31042480 Free PMC article.
-
Blocking ribosomal protein S6 phosphorylation inhibits podocyte hypertrophy and focal segmental glomerulosclerosis.Kidney Int. 2022 Jul;102(1):121-135. doi: 10.1016/j.kint.2022.02.037. Epub 2022 Apr 25. Kidney Int. 2022. PMID: 35483522 Free PMC article.
References
-
- United States Renal Data System : 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016
-
- D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 - PubMed
-
- Dantal J, Bigot E, Bogers W, Testa A, Kriaa F, Jacques Y, Hurault de Ligny B, Niaudet P, Charpentier B, Soulillou JP: Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N Engl J Med 330: 7–14, 1994 - PubMed
-
- Schachter ME, Monahan M, Radhakrishnan J, Crew J, Pollak M, Ratner L, Valeri AM, Stokes MB, Appel GB: Recurrent focal segmental glomerulosclerosis in the renal allograft: Single center experience in the era of modern immunosuppression. Clin Nephrol 74: 173–181, 2010 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

