Immunomodulatory glc/man-directed Dolichos lablab lectin (DLL) evokes anti-tumour response in vivo by counteracting angiogenic gene expressions
- PMID: 28268243
- PMCID: PMC5461098
- DOI: 10.1111/cei.12959
Immunomodulatory glc/man-directed Dolichos lablab lectin (DLL) evokes anti-tumour response in vivo by counteracting angiogenic gene expressions
Abstract
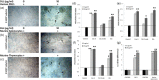
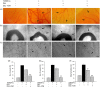
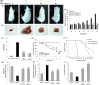
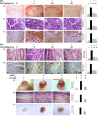
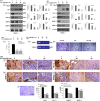
Neovascularization and jeopardized immunity has been critically emphasized for the establishment of malignant progression. Lectins are the diverse class of carbohydrate interacting proteins, having great potential as immunopotentiating and anti-cancer agents. The present investigation sought to demonstrate the anti-proliferative activity of Dolichos lablab lectin (DLL) encompassing immunomodulatory attributes. DLL specific to glucose and mannose carbohydrate moieties has been purified to homogeneity from the common dietary legume D. lablab. Results elucidated that DLL agglutinated blood cells non-specifically and displayed striking mitogenicity to human and murine lymphocytes in vitro with interleukin (IL)-2 production. The DLL-conditioned medium exerted cytotoxicity towards malignant cells and neoangiogenesis in vitro. Similarly, in-vivo anti-tumour investigation of DLL elucidated the regressed proliferation of ascitic and solid tumour cells, which was paralleled with blockade of tumour neovasculature. DLL-treated mice showed an up-regulated immunoregulatory cytokine IL-2 in contrast to severely declined levels in control mice. Mechanistic validation revealed that DLL has abrogated the microvessel formation by weakening the proangiogenic signals, specifically nuclear factor kappa B (NF-κB), hypoxia inducible factor 1α (HIF-1 α), matrix metalloproteinase (MMP)-2 and 9 and vascular endothelial growth factor (VEGF) in malignant cells leading to tumour regression. In summary, it is evident that the dietary lectin DLL potentially dampens the malignant establishment by mitigating neoangiogenesis and immune shutdown. For the first time, to our knowledge, this study illustrates the critical role of DLL as an immunostimulatory and anti-angiogenic molecule in cancer therapeutics.
Keywords: Dolichos lablab lectin; anti-tumour; immunomodulation; mitogenecity; tumour neovasculature.
© 2017 British Society for Immunology.
Figures

Similar articles
-
An efficient method for the purification and quantification of a galactose-specific lectin from vegetative tissues of Dolichos lablab.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Jan 15;861(2):209-17. doi: 10.1016/j.jchromb.2007.09.020. Epub 2007 Sep 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 17919997
-
Purification and characterization of Dolichos lablab lectin.Glycobiology. 1999 Feb;9(2):173-9. doi: 10.1093/glycob/9.2.173. Glycobiology. 1999. PMID: 9949194
-
New role of lupeol in reticence of angiogenesis, the cellular parameter of neoplastic progression in tumorigenesis models through altered gene expression.Biochem Biophys Res Commun. 2014 May 30;448(2):139-44. doi: 10.1016/j.bbrc.2014.04.090. Epub 2014 Apr 26. Biochem Biophys Res Commun. 2014. PMID: 24780400
-
Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth.Prog Biophys Mol Biol. 2013 Nov;113(2):333-54. doi: 10.1016/j.pbiomolbio.2013.10.001. Epub 2013 Oct 15. Prog Biophys Mol Biol. 2013. PMID: 24139944 Review.
-
The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties.Glycoconj J. 2013 Oct;30(7):641-57. doi: 10.1007/s10719-012-9464-4. Epub 2013 Jan 9. Glycoconj J. 2013. PMID: 23299509 Free PMC article. Review.
Cited by
-
Co-Targeting Tumor Angiogenesis and Immunosuppressive Tumor Microenvironment: A Perspective in Ethnopharmacology.Front Pharmacol. 2022 Jun 15;13:886198. doi: 10.3389/fphar.2022.886198. eCollection 2022. Front Pharmacol. 2022. PMID: 35784750 Free PMC article. Review.
-
Immunomodulatory Effects of Jacalin, a Dietary Plant Lectin on the Peripheral Blood Mononuclear Cells (PBMCs).Appl Biochem Biotechnol. 2022 Jan;194(1):587-599. doi: 10.1007/s12010-021-03722-6. Epub 2021 Oct 28. Appl Biochem Biotechnol. 2022. PMID: 34709568
-
Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity.Medchemcomm. 2019 Jan 14;10(3):390-398. doi: 10.1039/c8md00508g. eCollection 2019 Mar 1. Medchemcomm. 2019. PMID: 30996857 Free PMC article.
-
A Carbohydrate-Binding Protein from the Edible Lablab Beans Effectively Blocks the Infections of Influenza Viruses and SARS-CoV-2.Cell Rep. 2020 Aug 11;32(6):108016. doi: 10.1016/j.celrep.2020.108016. Epub 2020 Jul 24. Cell Rep. 2020. PMID: 32755598 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

