Acute death of astrocytes in blast-exposed rat organotypic hippocampal slice cultures
- PMID: 28264063
- PMCID: PMC5338800
- DOI: 10.1371/journal.pone.0173167
Acute death of astrocytes in blast-exposed rat organotypic hippocampal slice cultures
Abstract
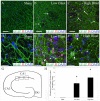
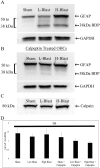
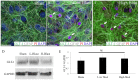
Blast traumatic brain injury (bTBI) affects civilians, soldiers, and veterans worldwide and presents significant health concerns. The mechanisms of neurodegeneration following bTBI remain elusive and current therapies are largely ineffective. It is important to better characterize blast-evoked cellular changes and underlying mechanisms in order to develop more effective therapies. In the present study, our group utilized rat organotypic hippocampal slice cultures (OHCs) as an in vitro system to model bTBI. OHCs were exposed to either 138 ± 22 kPa (low) or 273 ± 23 kPa (high) overpressures using an open-ended helium-driven shock tube, or were assigned to sham control group. At 2 hours (h) following injury, we have characterized the astrocytic response to a blast overpressure. Immunostaining against the astrocytic marker glial fibrillary acidic protein (GFAP) revealed acute shearing and morphological changes in astrocytes, including clasmatodendrosis. Moreover, overlap of GFAP immunostaining and propidium iodide (PI) indicated astrocytic death. Quantification of the number of dead astrocytes per counting area in the hippocampal cornu Ammonis 1 region (CA1), demonstrated a significant increase in dead astrocytes in the low- and high-blast, compared to sham control OHCs. However only a small number of GFAP-expressing astrocytes were co-labeled with the apoptotic marker Annexin V, suggesting necrosis as the primary type of cell death in the acute phase following blast exposure. Moreover, western blot analyses revealed calpain mediated breakdown of GFAP. The dextran exclusion additionally indicated membrane disruption as a potential mechanism of acute astrocytic death. Furthermore, although blast exposure did not evoke significant changes in glutamate transporter 1 (GLT-1) expression, loss of GLT-1-expressing astrocytes suggests dysregulation of glutamate uptake following injury. Our data illustrate the profound effect of blast overpressure on astrocytes in OHCs at 2 h following injury and suggest increased calpain activity and membrane disruption as potential underlying mechanisms.
Conflict of interest statement
Figures


Similar articles
-
Effects of blast overpressure on neurons and glial cells in rat organotypic hippocampal slice cultures.Front Neurol. 2015 Feb 12;6:20. doi: 10.3389/fneur.2015.00020. eCollection 2015. Front Neurol. 2015. PMID: 25729377 Free PMC article.
-
Simulated blast overpressure induces specific astrocyte injury in an ex vivo brain slice model.PLoS One. 2017 Apr 12;12(4):e0175396. doi: 10.1371/journal.pone.0175396. eCollection 2017. PLoS One. 2017. PMID: 28403239 Free PMC article.
-
Modeling clinically relevant blast parameters based on scaling principles produces functional & histological deficits in rats.Exp Neurol. 2013 Oct;248:520-9. doi: 10.1016/j.expneurol.2013.07.008. Epub 2013 Jul 20. Exp Neurol. 2013. PMID: 23876514
-
Control of the phosphorylation of the astrocyte marker glial fibrillary acidic protein (GFAP) in the immature rat hippocampus by glutamate and calcium ions: possible key factor in astrocytic plasticity.Braz J Med Biol Res. 1997 Mar;30(3):325-38. doi: 10.1590/s0100-879x1997000300005. Braz J Med Biol Res. 1997. PMID: 9246230 Review.
-
The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma.Int J Mol Sci. 2024 Jan 17;25(2):1150. doi: 10.3390/ijms25021150. Int J Mol Sci. 2024. PMID: 38256223 Free PMC article. Review.
Cited by
-
Organotypic slice culture based on in ovo electroporation for chicken embryonic central nervous system.J Cell Mol Med. 2019 Mar;23(3):1813-1826. doi: 10.1111/jcmm.14080. Epub 2018 Dec 18. J Cell Mol Med. 2019. PMID: 30565384 Free PMC article.
-
An Overview on the Differential Interplay Among Neurons-Astrocytes-Microglia in CA1 and CA3 Hippocampus in Hypoxia/Ischemia.Front Cell Neurosci. 2020 Nov 11;14:585833. doi: 10.3389/fncel.2020.585833. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33262692 Free PMC article. Review.
-
Blast-Induced Traumatic Brain Injury Triggered by Moderate Intensity Shock Wave Using a Modified Experimental Model of Injury in Mice.Chin Med J (Engl). 2018 Oct 20;131(20):2447-2460. doi: 10.4103/0366-6999.243558. Chin Med J (Engl). 2018. PMID: 30334530 Free PMC article.
-
Glial Activation in the Thalamus Contributes to Vestibulomotor Deficits Following Blast-Induced Neurotrauma.Front Neurol. 2020 Jul 15;11:618. doi: 10.3389/fneur.2020.00618. eCollection 2020. Front Neurol. 2020. PMID: 32760340 Free PMC article.
-
Increasing cellular lifespan with a flow system in organotypic culture of the Laterodorsal Tegmentum (LDT).Sci Rep. 2019 Feb 6;9(1):1486. doi: 10.1038/s41598-018-37606-3. Sci Rep. 2019. PMID: 30728375 Free PMC article.
References
-
- Warden D. Military TBI during the Iraq and Afghanistan wars. The Journal of head trauma rehabilitation. 2006;21(5):398–402. Epub 2006/09/20. - PubMed
-
- Shively SB, Perl DP. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military—past, present, and future. The Journal of head trauma rehabilitation. 2012;27(3):234–9. Epub 2012/05/11. - PubMed
-
- Bell RS, Vo AH, Neal CJ, Tigno J, Roberts R, Mossop C, et al. Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. The Journal of trauma. 2009;66(4 Suppl):S104–11. Epub 2009/06/12. 10.1097/TA.0b013e31819d88c8 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

