Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice
- PMID: 28263708
- PMCID: PMC5338920
- DOI: 10.7554/eLife.18128
Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice
Abstract
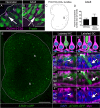
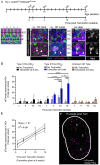
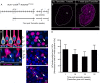
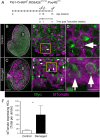
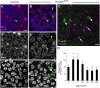
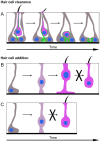
Vestibular hair cells in the inner ear encode head movements and mediate the sense of balance. These cells undergo cell death and replacement (turnover) throughout life in non-mammalian vertebrates. However, there is no definitive evidence that this process occurs in mammals. We used fate-mapping and other methods to demonstrate that utricular type II vestibular hair cells undergo turnover in adult mice under normal conditions. We found that supporting cells phagocytose both type I and II hair cells. Plp1-CreERT2-expressing supporting cells replace type II hair cells. Type I hair cells are not restored by Plp1-CreERT2-expressing supporting cells or by Atoh1-CreERTM-expressing type II hair cells. Destruction of hair cells causes supporting cells to generate 6 times as many type II hair cells compared to normal conditions. These findings expand our understanding of sensorineural plasticity in adult vestibular organs and further elucidate the roles that supporting cells serve during homeostasis and after injury.
Keywords: developmental biology; hair cell; mouse; neuroscience; regeneration; stem cells; supporting cell; turnover; utricle; vestibular.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
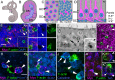
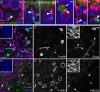
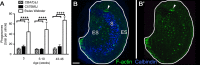
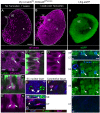
Similar articles
-
Atoh1 is required in supporting cells for regeneration of vestibular hair cells in adult mice.Hear Res. 2020 Jan;385:107838. doi: 10.1016/j.heares.2019.107838. Epub 2019 Nov 7. Hear Res. 2020. PMID: 31751832 Free PMC article.
-
Characterization of Adult Vestibular Organs in 11 CreER Mouse Lines.J Assoc Res Otolaryngol. 2018 Aug;19(4):381-399. doi: 10.1007/s10162-018-0676-6. Epub 2018 Jun 4. J Assoc Res Otolaryngol. 2018. PMID: 29869046 Free PMC article.
-
Simultaneous gentamicin-mediated damage and Atoh1 overexpression promotes hair cell regeneration in the neonatal mouse utricle.Exp Cell Res. 2021 Jan 1;398(1):112395. doi: 10.1016/j.yexcr.2020.112395. Epub 2020 Dec 3. Exp Cell Res. 2021. PMID: 33279477
-
Regeneration of hair cells in the mammalian vestibular system.Front Med. 2016 Jun;10(2):143-51. doi: 10.1007/s11684-016-0451-1. Epub 2016 May 17. Front Med. 2016. PMID: 27189205 Review.
-
Ongoing cell death and immune influences on regeneration in the vestibular sensory organs.Ann N Y Acad Sci. 2001 Oct;942:34-45. doi: 10.1111/j.1749-6632.2001.tb03733.x. Ann N Y Acad Sci. 2001. PMID: 11710476 Review.
Cited by
-
Fgf8 genetic labeling reveals the early specification of vestibular hair cell type in mouse utricle.Development. 2020 Nov 19;147(22):dev192849. doi: 10.1242/dev.192849. Development. 2020. PMID: 33046506 Free PMC article.
-
Regenerating hair cells in vestibular sensory epithelia from humans.Elife. 2018 Jul 18;7:e34817. doi: 10.7554/eLife.34817. Elife. 2018. PMID: 30019672 Free PMC article.
-
Atoh1 is required in supporting cells for regeneration of vestibular hair cells in adult mice.Hear Res. 2020 Jan;385:107838. doi: 10.1016/j.heares.2019.107838. Epub 2019 Nov 7. Hear Res. 2020. PMID: 31751832 Free PMC article.
-
Development of hair cell phenotype and calyx nerve terminals in the neonatal mouse utricle.J Comp Neurol. 2019 Aug 1;527(11):1913-1928. doi: 10.1002/cne.24658. Epub 2019 Feb 22. J Comp Neurol. 2019. PMID: 30724338 Free PMC article.
-
Sensorineural correlates of failed functional recovery after natural regeneration of vestibular hair cells in adult mice.Front Neurol. 2024 Mar 8;15:1322647. doi: 10.3389/fneur.2024.1322647. eCollection 2024. Front Neurol. 2024. PMID: 38523617 Free PMC article.
References
-
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. Journal of Neuroscience. 2006;26:7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. - DOI - PMC - PubMed
-
- Anttonen T, Belevich I, Kirjavainen A, Laos M, Brakebusch C, Jokitalo E, Pirvola U. How to bury the dead: elimination of apoptotic hair cells from the hearing organ of the mouse. Journal of the Association for Research in Otolaryngology. 2014;15:975–992. doi: 10.1007/s10162-014-0480-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

