Potential targeting of B7-H4 for the treatment of cancer
- PMID: 28258701
- PMCID: PMC5630270
- DOI: 10.1111/imr.12530
Potential targeting of B7-H4 for the treatment of cancer
Abstract
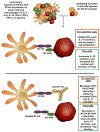
Observations noting the presence of white blood cell infiltrates within tumors date back more than a century, however the cellular and molecular mechanisms regulating tumor immunity continue to be elucidated. The recent successful use of monoclonal antibodies to block immune regulatory pathways to enhance tumor-specific immune responses for the treatment of cancer has encouraged the identification of additional immune regulatory receptor/ligand pathways. Over the past several years, a growing body of data has identified B7-H4 (VTCN1/B7x/B7S1) as a potential therapeutic target for the treatment of cancer. The potential clinical significance of B7-H4 is supported by the high levels of B7-H4 expression found in numerous tumor tissues and correlation of the level of expression on tumor cells with adverse clinical and pathologic features, including tumor aggressiveness. The biological activity of B7-H4 has been associated with decreased inflammatory CD4+ T-cell responses and a correlation between B7-H4-expressing tumor-associated macrophages and FoxP3+ regulatory T cells (Tregs) within the tumor microenvironment. Since B7-H4 is expressed on tumor cells and tumor-associated macrophages in various cancer types, therapeutic blockade of B7-H4 could favorably alter the tumor microenvironment allowing for antigen-specific clearance tumor cells. The present review highlights the therapeutic potential of targeting B7-H4.
Keywords: B7-H4; B7-H4 receptor; CD4+ T cell; cancer; co-stimulatory/co-inhibitory molecule; regulatory T cell.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors do not have any conflicts of interest regarding this work.
Figures
Similar articles
-
Could B7-H4 serve as a target to activate anti-cancer immunity?Int Immunopharmacol. 2016 Sep;38:97-103. doi: 10.1016/j.intimp.2016.05.020. Epub 2016 Jun 1. Int Immunopharmacol. 2016. PMID: 27258187 Review.
-
B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration.Acta Histochem. 2014 Sep;116(7):1163-8. doi: 10.1016/j.acthis.2014.06.003. Epub 2014 Jul 19. Acta Histochem. 2014. PMID: 25053455
-
Induced expression of B7-H4 on the surface of lung cancer cell by the tumor-associated macrophages: a potential mechanism of immune escape.Cancer Lett. 2012 Apr 1;317(1):99-105. doi: 10.1016/j.canlet.2011.11.017. Epub 2011 Nov 20. Cancer Lett. 2012. PMID: 22108530
-
Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses.Cancer Res. 2013 Aug 1;73(15):4820-9. doi: 10.1158/0008-5472.CAN-12-3457. Epub 2013 May 30. Cancer Res. 2013. PMID: 23722540 Free PMC article.
-
B7-H4, a promising target for immunotherapy.Cell Immunol. 2020 Jan;347:104008. doi: 10.1016/j.cellimm.2019.104008. Epub 2019 Nov 4. Cell Immunol. 2020. PMID: 31733822 Review.
Cited by
-
Emerging Therapeutic Targets and Drug Resistance Mechanisms in Immunotherapy of Hematological Malignancies.Cancers (Basel). 2023 Dec 8;15(24):5765. doi: 10.3390/cancers15245765. Cancers (Basel). 2023. PMID: 38136311 Free PMC article. Review.
-
Exploiting innate immunity for cancer immunotherapy.Mol Cancer. 2023 Nov 27;22(1):187. doi: 10.1186/s12943-023-01885-w. Mol Cancer. 2023. PMID: 38008741 Free PMC article. Review.
-
B7 Family Members in Pancreatic Ductal Adenocarcinoma: Attractive Targets for Cancer Immunotherapy.Int J Mol Sci. 2022 Nov 30;23(23):15005. doi: 10.3390/ijms232315005. Int J Mol Sci. 2022. PMID: 36499340 Free PMC article. Review.
-
A new immune checkpoint-associated nine-gene signature for prognostic prediction of glioblastoma.Medicine (Baltimore). 2023 Mar 3;102(9):e33150. doi: 10.1097/MD.0000000000033150. Medicine (Baltimore). 2023. PMID: 36862886 Free PMC article.
-
A Predictor of Pathological Complete Response to Neoadjuvant Chemotherapy Stratifies Triple Negative Breast Cancer Patients with High Risk of Recurrence.Sci Rep. 2019 Oct 16;9(1):14863. doi: 10.1038/s41598-019-51335-1. Sci Rep. 2019. PMID: 31619719 Free PMC article.
References
-
- Dong C, Nurieva RI, Prasad DV. Immune regulation by novel costimulatory molecules. Immunol Res. 2003;28:39–48. - PubMed
-
- Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

