Preferential Lineage-Specific Differentiation of Osteoblast-Derived Induced Pluripotent Stem Cells into Osteoprogenitors
- PMID: 28250775
- PMCID: PMC5303871
- DOI: 10.1155/2017/1513281
Preferential Lineage-Specific Differentiation of Osteoblast-Derived Induced Pluripotent Stem Cells into Osteoprogenitors
Abstract
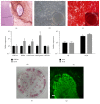
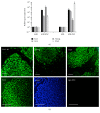
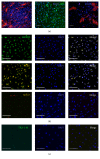
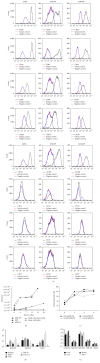
While induced pluripotent stem cells (iPSCs) hold great clinical promise, one hurdle that remains is the existence of a parental germ-layer memory in reprogrammed cells leading to preferential differentiation fates. While it is problematic for generating cells vastly different from the reprogrammed cells' origins, it could be advantageous for the reliable generation of germ-layer specific cell types for future therapeutic use. Here we use human osteoblast-derived iPSCs (hOB-iPSCs) to generate induced osteoprogenitors (iOPs). Osteoblasts were successfully reprogrammed and demonstrated by endogenous upregulation of Oct4, Sox2, Nanog, TRA-1-81, TRA-16-1, SSEA3, and confirmatory hPSC Scorecard Algorithmic Assessment. The hOB-iPSCs formed embryoid bodies with cells of ectoderm and mesoderm but have low capacity to form endodermal cells. Differentiation into osteoprogenitors occurred within only 2-6 days, with a population doubling rate of less than 24 hrs; however, hOB-iPSC derived osteoprogenitors were only able to form osteogenic and chondrogenic cells but not adipogenic cells. Consistent with this, hOB-iOPs were found to have higher methylation of PPARγ but similar levels of methylation on the RUNX2 promoter. These data demonstrate that iPSCs can be generated from human osteoblasts, but variant methylation patterns affect their differentiation capacities. Therefore, epigenetic memory can be exploited for efficient generation of clinically relevant quantities of osteoprogenitor cells.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures

Similar articles
-
Generation of two induced pluripotent stem cells lines from a Mucopolysaccharydosis IIIB (MPSIIIB) patient.Stem Cell Res. 2018 Dec;33:180-184. doi: 10.1016/j.scr.2018.10.019. Epub 2018 Nov 1. Stem Cell Res. 2018. PMID: 30408744
-
Efficient hematopoietic redifferentiation of induced pluripotent stem cells derived from primitive murine bone marrow cells.Stem Cells Dev. 2012 Mar 20;21(5):689-701. doi: 10.1089/scd.2011.0010. Epub 2011 Aug 24. Stem Cells Dev. 2012. PMID: 21732815
-
Generation of Induced Pluripotent Stem Cells from Human Epidermal Keratinocytes.Cell Reprogram. 2018 Dec;20(6):356-364. doi: 10.1089/cell.2018.0035. Epub 2018 Nov 2. Cell Reprogram. 2018. PMID: 30388030
-
Susceptibility of Human Oral Squamous Cell Carcinoma (OSCC) H103 and H376 cell lines to Retroviral OSKM mediated reprogramming.PeerJ. 2017 Apr 13;5:e3174. doi: 10.7717/peerj.3174. eCollection 2017. PeerJ. 2017. PMID: 28417059 Free PMC article.
-
Induced Pluripotent Stem Cells: A New Frontier for Stem Cells in Dentistry.J Dent Res. 2015 Nov;94(11):1508-15. doi: 10.1177/0022034515599769. Epub 2015 Aug 18. J Dent Res. 2015. PMID: 26285811 Review.
Cited by
-
Cell viability assessed in a reproducible model of human osteoblasts derived from human adipose-derived stem cells.PLoS One. 2018 Apr 11;13(4):e0194847. doi: 10.1371/journal.pone.0194847. eCollection 2018. PLoS One. 2018. PMID: 29641603 Free PMC article.
-
Reprogramming of Mouse Calvarial Osteoblasts into Induced Pluripotent Stem Cells.Stem Cells Int. 2018 Mar 12;2018:5280793. doi: 10.1155/2018/5280793. eCollection 2018. Stem Cells Int. 2018. PMID: 29721022 Free PMC article.
-
Sequential transfection of RUNX2/SP7 and ATF4 coated onto dexamethasone-loaded nanospheresenhances osteogenesis.Sci Rep. 2018 Jan 23;8(1):1447. doi: 10.1038/s41598-018-19824-x. Sci Rep. 2018. PMID: 29362501 Free PMC article.
-
Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach.World J Stem Cells. 2020 Jan 26;12(1):25-34. doi: 10.4252/wjsc.v12.i1.25. World J Stem Cells. 2020. PMID: 32110273 Free PMC article. Review.
-
A Review of Recent Advances in 3D Bioprinting With an Eye on Future Regenerative Therapies in Veterinary Medicine.Front Vet Sci. 2021 Feb 16;7:584193. doi: 10.3389/fvets.2020.584193. eCollection 2020. Front Vet Sci. 2021. PMID: 33665213 Free PMC article. Review.
References
-
- Musante D. B., Firtha M. E., Atkinson B. L., Hahn R., Ryaby J. T., Linovitz R. J. Clinical evaluation of an allogeneic bone matrix containing viable osteogenic cells in patients undergoing one- and two-level posterolateral lumbar arthrodesis with decompressive laminectomy. Journal of Orthopaedic Surgery and Research. 2016;11(1, article 63) doi: 10.1186/s13018-016-0392-z. - DOI - PMC - PubMed
-
- Vanichkachorn J., Peppers T., Bullard D., Stanley S. K., Linovitz R. J., Ryaby J. T. A prospective clinical and radiographic 12-month outcome study of patients undergoing single-level anterior cervical discectomy and fusion for symptomatic cervical degenerative disc disease utilizing a novel viable allogeneic, cancellous, bone matrix (trinity evolution™) with a comparison to historical controls. European Spine Journal. 2016;25(7):2233–2238. doi: 10.1007/s00586-016-4414-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

