Regulation of DENND3, the exchange factor for the small GTPase Rab12 through an intramolecular interaction
- PMID: 28249939
- PMCID: PMC5409492
- DOI: 10.1074/jbc.M116.772434
Regulation of DENND3, the exchange factor for the small GTPase Rab12 through an intramolecular interaction
Abstract
The Rab family of small GTPases functions in multiple aspects of cellular membrane trafficking. Proteins bearing a
Keywords: DENN domain; DENND3; Rab12; autophagy; guanine nucleotide exchange factor (GEF); intramolecular interaction; membrane trafficking; oligomerization; phosphorylation; phosphotyrosine.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

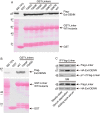
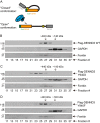

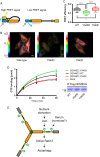
Comment in
-
Into the linker's DENN: A tyrosine's control of autophagy.J Biol Chem. 2017 Apr 28;292(17):7283-7284. doi: 10.1074/jbc.H116.772434. Epub 2017 Apr 28. J Biol Chem. 2017. PMID: 28455410 Free PMC article.
Similar articles
-
A PH-like domain of the Rab12 guanine nucleotide exchange factor DENND3 binds actin and is required for autophagy.J Biol Chem. 2018 Mar 23;293(12):4566-4574. doi: 10.1074/jbc.RA117.001446. Epub 2018 Jan 19. J Biol Chem. 2018. PMID: 29352104 Free PMC article.
-
Phosphorylation of the exchange factor DENND3 by ULK in response to starvation activates Rab12 and induces autophagy.EMBO Rep. 2015 Jun;16(6):709-18. doi: 10.15252/embr.201440006. Epub 2015 Apr 29. EMBO Rep. 2015. PMID: 25925668 Free PMC article.
-
Dennd3 functions as a guanine nucleotide exchange factor for small GTPase Rab12 in mouse embryonic fibroblasts.J Biol Chem. 2014 May 16;289(20):13986-95. doi: 10.1074/jbc.M113.546689. Epub 2014 Apr 9. J Biol Chem. 2014. PMID: 24719330 Free PMC article.
-
Multiple Types of Guanine Nucleotide Exchange Factors (GEFs) for Rab Small GTPases.Cell Struct Funct. 2016 Jul 9;41(2):61-79. doi: 10.1247/csf.16008. Epub 2016 May 27. Cell Struct Funct. 2016. PMID: 27246931 Review.
-
DENN domain proteins: regulators of Rab GTPases.J Biol Chem. 2011 Apr 22;286(16):13791-800. doi: 10.1074/jbc.R110.217067. Epub 2011 Feb 17. J Biol Chem. 2011. PMID: 21330364 Free PMC article. Review.
Cited by
-
A new actin-binding domain glues autophagy together.J Biol Chem. 2018 Mar 23;293(12):4575-4576. doi: 10.1074/jbc.H118.002041. J Biol Chem. 2018. PMID: 29572327 Free PMC article.
-
Genomic Characterization of Prostatic Basal Cell Carcinoma.Am J Pathol. 2023 Jan;193(1):4-10. doi: 10.1016/j.ajpath.2022.09.010. Epub 2022 Oct 26. Am J Pathol. 2023. PMID: 36309102 Free PMC article.
-
Into the linker's DENN: A tyrosine's control of autophagy.J Biol Chem. 2017 Apr 28;292(17):7283-7284. doi: 10.1074/jbc.H116.772434. Epub 2017 Apr 28. J Biol Chem. 2017. PMID: 28455410 Free PMC article.
-
DENND3 p.L708V activating variant is involved in the pathogenesis of hereditary hemochromatosis via the RAB12/TFR2 signaling pathway.Hepatol Int. 2023 Jun;17(3):648-661. doi: 10.1007/s12072-022-10474-w. Epub 2023 Feb 2. Hepatol Int. 2023. PMID: 36729283
-
Development of a Bioinformatics Framework for Identification and Validation of Genomic Biomarkers and Key Immunopathology Processes and Controllers in Infectious and Non-infectious Severe Inflammatory Response Syndrome.Front Immunol. 2020 Mar 31;11:380. doi: 10.3389/fimmu.2020.00380. eCollection 2020. Front Immunol. 2020. PMID: 32318053 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

