Store-operated calcium entry is essential for glial calcium signalling in CNS white matter
- PMID: 28247021
- PMCID: PMC5585307
- DOI: 10.1007/s00429-017-1380-8
Store-operated calcium entry is essential for glial calcium signalling in CNS white matter
Abstract
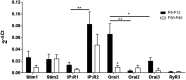
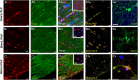
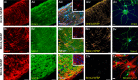
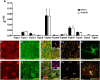
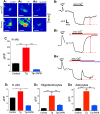
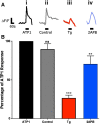
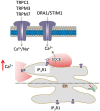
'Calcium signalling' is the ubiquitous response of glial cells to multiple extracellular stimuli. The primary mechanism of glial calcium signalling is by release of calcium from intracellular stores of the endoplasmic reticulum (ER). Replenishment of ER Ca2+ stores relies on store-operated calcium entry (SOCE). However, despite the importance of calcium signalling in glial cells, little is known about their mechanisms of SOCE. Here, we investigated SOCE in glia of the mouse optic nerve, a typical CNS white matter tract that comprises bundles of myelinated axons and the oligodendrocytes and astrocytes that support them. Using quantitative RT-PCR, we identified Orai1 channels, both Stim1 and Stim2, and the transient receptor potential M3 channel (TRPM3) as the primary channels for SOCE in the optic nerve, and their expression in both astrocytes and oligodendrocytes was demonstrated by immunolabelling of optic nerve sections and cultures. The functional importance of SOCE was demonstrated by fluo-4 calcium imaging on isolated intact optic nerves and optic nerve cultures. Removal of extracellular calcium ([Ca2+]o) resulted in a marked depletion of glial cytosolic calcium ([Ca2+]i), which recovered rapidly on restoration of [Ca2+]o via SOCE. 2-aminoethoxydiphenylborane (2APB) significantly decreased SOCE and severely attenuated ATP-mediated calcium signalling. The results provide evidence that Orai/Stim and TRPM3 are important components of the 'calcium toolkit' that underpins SOCE and the sustainability of calcium signalling in white matter glia.
Keywords: Astrocyte; CRAC; Calcium signalling; Glia; Oligodendrocyte; Store-operated calcium channel; TRP channel; White matter.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Figures
Similar articles
-
Interplay between ER Ca2+ Binding Proteins, STIM1 and STIM2, Is Required for Store-Operated Ca2+ Entry.Int J Mol Sci. 2018 May 19;19(5):1522. doi: 10.3390/ijms19051522. Int J Mol Sci. 2018. PMID: 29783744 Free PMC article.
-
Cross-talk between N-terminal and C-terminal domains in stromal interaction molecule 2 (STIM2) determines enhanced STIM2 sensitivity.J Biol Chem. 2019 Apr 19;294(16):6318-6332. doi: 10.1074/jbc.RA118.006801. Epub 2019 Mar 1. J Biol Chem. 2019. PMID: 30824535 Free PMC article.
-
Store-operated Ca2+ entry is not required for fertilization-induced Ca2+ signaling in mouse eggs.Cell Calcium. 2017 Jul;65:63-72. doi: 10.1016/j.ceca.2017.02.004. Epub 2017 Feb 11. Cell Calcium. 2017. PMID: 28222911 Free PMC article.
-
Store-Independent Orai Channels Regulated by STIM.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
Cited by
-
Calcium release-activated calcium channels and pain.Cell Calcium. 2018 Sep;74:180-185. doi: 10.1016/j.ceca.2018.07.009. Epub 2018 Jul 29. Cell Calcium. 2018. PMID: 30096536 Free PMC article. Review.
-
TRPP2 associates with STIM1 to regulate cerebral vasoconstriction and enhance high salt intake-induced hypertensive cerebrovascular spasm.Hypertens Res. 2019 Dec;42(12):1894-1904. doi: 10.1038/s41440-019-0324-5. Epub 2019 Sep 20. Hypertens Res. 2019. PMID: 31541223
-
Validation of impaired Transient Receptor Potential Melastatin 3 ion channel activity in natural killer cells from Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis patients.Mol Med. 2019 Apr 23;25(1):14. doi: 10.1186/s10020-019-0083-4. Mol Med. 2019. PMID: 31014226 Free PMC article.
-
Ca2+-induced myelin pathology precedes axonal spheroid formation and is mediated in part by store-operated Ca2+ entry after spinal cord injury.Neural Regen Res. 2023 Dec;18(12):2720-2726. doi: 10.4103/1673-5374.373656. Neural Regen Res. 2023. PMID: 37449636 Free PMC article.
-
Transmembrane Prolyl 4-Hydroxylase is a Novel Regulator of Calcium Signaling in Astrocytes.eNeuro. 2021 Jan 8;8(1):ENEURO.0253-20.2020. doi: 10.1523/ENEURO.0253-20.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33298456 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

