Motor neuron intrinsic and extrinsic mechanisms contribute to the pathogenesis of FUS-associated amyotrophic lateral sclerosis
- PMID: 28243725
- PMCID: PMC5427169
- DOI: 10.1007/s00401-017-1687-9
Motor neuron intrinsic and extrinsic mechanisms contribute to the pathogenesis of FUS-associated amyotrophic lateral sclerosis
Abstract
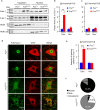
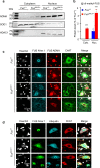
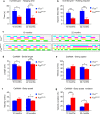
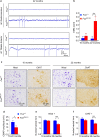
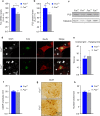
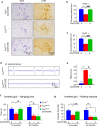
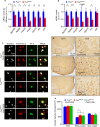
Motor neuron-extrinsic mechanisms have been shown to participate in the pathogenesis of ALS-SOD1, one familial form of amyotrophic lateral sclerosis (ALS). It remains unclear whether such mechanisms contribute to other familial forms, such as TDP-43 and FUS-associated ALS. Here, we characterize a single-copy mouse model of ALS-FUS that conditionally expresses a disease-relevant truncating FUS mutant from the endogenous murine Fus gene. We show that these mice, but not mice heterozygous for a Fus null allele, develop similar pathology as ALS-FUS patients and a mild motor neuron phenotype. Most importantly, CRE-mediated rescue of the Fus mutation within motor neurons prevented degeneration of motor neuron cell bodies, but only delayed appearance of motor symptoms. Indeed, we observed downregulation of multiple myelin-related genes, and increased numbers of oligodendrocytes in the spinal cord supporting their contribution to behavioral deficits. In all, we show that mutant FUS triggers toxic events in both motor neurons and neighboring cells to elicit motor neuron disease.
Keywords: Amyotrophic lateral sclerosis; Fronto-temporal dementia; Mouse models; Non-cell autonomous mechanisms; RNA-binding proteins.
Figures
Similar articles
-
ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function.Nat Commun. 2016 Feb 4;7:10465. doi: 10.1038/ncomms10465. Nat Commun. 2016. PMID: 26842965 Free PMC article.
-
Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis.PLoS One. 2012;7(4):e35050. doi: 10.1371/journal.pone.0035050. Epub 2012 Apr 6. PLoS One. 2012. PMID: 22493728 Free PMC article.
-
Vascular regression precedes motor neuron loss in the FUS (1-359) ALS mouse model.Dis Model Mech. 2019 Aug 13;12(8):dmm040238. doi: 10.1242/dmm.040238. Dis Model Mech. 2019. PMID: 31383794 Free PMC article.
-
[The FUS protein: Physiological functions and a role in amyotrophic lateral sclerosis].Mol Biol (Mosk). 2017 May-Jun;51(3):387-399. doi: 10.7868/S0026898417020094. Mol Biol (Mosk). 2017. PMID: 28707655 Review. Russian.
-
Animal Models of FUS-Proteinopathy: A Systematic Review.Biochemistry (Mosc). 2024 Jan;89(Suppl 1):S34-S56. doi: 10.1134/S0006297924140037. Biochemistry (Mosc). 2024. PMID: 38621743 Review.
Cited by
-
Endogenous TDP-43 mislocalization in a novel knock-in mouse model reveals DNA repair impairment, inflammation, and neuronal senescence.Res Sq [Preprint]. 2024 Mar 20:rs.3.rs-3879966. doi: 10.21203/rs.3.rs-3879966/v1. Res Sq. 2024. PMID: 38343852 Free PMC article. Preprint.
-
Integrated identification of key genes and pathways in Alzheimer's disease via comprehensive bioinformatical analyses.Hereditas. 2019 Jul 16;156:25. doi: 10.1186/s41065-019-0101-0. eCollection 2019. Hereditas. 2019. PMID: 31346329 Free PMC article.
-
Animal models of neurodegenerative diseases.Nat Neurosci. 2018 Oct;21(10):1370-1379. doi: 10.1038/s41593-018-0236-8. Epub 2018 Sep 24. Nat Neurosci. 2018. PMID: 30250265 Free PMC article. Review.
-
ALS/FTD-Linked Mutation in FUS Suppresses Intra-axonal Protein Synthesis and Drives Disease Without Nuclear Loss-of-Function of FUS.Neuron. 2018 Nov 21;100(4):816-830.e7. doi: 10.1016/j.neuron.2018.09.044. Epub 2018 Oct 18. Neuron. 2018. PMID: 30344044 Free PMC article.
-
Arginine Methyltransferase PRMT8 Provides Cellular Stress Tolerance in Aging Motoneurons.J Neurosci. 2018 Aug 29;38(35):7683-7700. doi: 10.1523/JNEUROSCI.3389-17.2018. Epub 2018 Jul 27. J Neurosci. 2018. PMID: 30054395 Free PMC article.
References
-
- Belly A, Moreau-Gachelin F, Sadoul R, Goldberg Y. Delocalization of the multifunctional RNA splicing factor TLS/FUS in hippocampal neurones: exclusion from the nucleus and accumulation in dendritic granules and spine heads. Neurosci Lett. 2005;379:152–157. doi: 10.1016/j.neulet.2004.12.071. - DOI - PubMed
-
- Bergemalm D, Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Rehnmark A, Marklund SL. Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models. J Neurosci. 2006;26:4147–4154. doi: 10.1523/JNEUROSCI.5461-05.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

