Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction
- PMID: 28236106
- PMCID: PMC5401699
- DOI: 10.1007/s10815-017-0879-5
Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction
Erratum in
-
Correction to: Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction.J Assist Reprod Genet. 2019 Aug;36(8):1759-1760. doi: 10.1007/s10815-019-01512-y. J Assist Reprod Genet. 2019. PMID: 31273586 Free PMC article.
Abstract
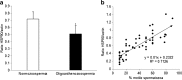
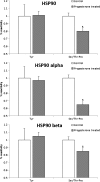
Purpose: The aims of this paper were to study whether heat shock protein 90 (HSP90) is a regulator of sperm functions and to determine its association with oligoasthenozoospermia.
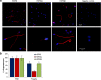
Methods: The levels of HSP90 in sperm lysates were measured by ELISA. Localization of HSP90 and its isoforms was evaluated by immunofluorescence. Sperm motility and kinetics were assessed by computer-assisted sperm analysis. Acrosome reaction was determined by lectin staining.
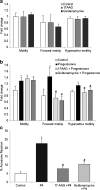
Results: The levels of HSP90 were lower in oligoasthenozoospermic men and correlated positively with the number of motile spermatozoa. In capacitated human spermatozoa, HSP90α was mostly found in residual nuclear envelope, and the HSP90β isoform was higher in the flagella. Inhibition of HSP90 by geldanamycin or 17-AAG did not affect basal motility, but suppressed progesterone-mediated forward progressive motility, hyperactivation and acrosome reaction. Progesterone treatment dephosphorylated both HSP90α and HSP90β at Ser/Thr-Pro residues, but not Tyr residues.
Conclusion: HSP90 levels are downregulated in oligoasthenozoospermia, and its functional inhibition attenuates progesterone-mediated sperm motility and acrosome reaction.
Keywords: Acrosome reaction; HSP90; Heat shock protein; Human sperm; Motility; Oligoasthenozoospermia; Phosphorylation; Progesterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hsp90 modulates human sperm capacitation via the Erk1/2 and p38 MAPK signaling pathways.Reprod Biol Endocrinol. 2021 Mar 4;19(1):39. doi: 10.1186/s12958-021-00723-2. Reprod Biol Endocrinol. 2021. PMID: 33663544 Free PMC article.
-
Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction.Mol Cell Endocrinol. 2013 Jul 15;374(1-2):82-91. doi: 10.1016/j.mce.2013.04.005. Epub 2013 Apr 24. Mol Cell Endocrinol. 2013. PMID: 23623968
-
Heat shock protein 90 has roles in intracellular calcium homeostasis, protein tyrosine phosphorylation regulation, and progesterone-responsive sperm function in human sperm.PLoS One. 2014 Dec 26;9(12):e115841. doi: 10.1371/journal.pone.0115841. eCollection 2014. PLoS One. 2014. PMID: 25541943 Free PMC article.
-
Pathophysiology of sperm motility.Front Biosci. 2006 May 1;11:1433-47. doi: 10.2741/1894. Front Biosci. 2006. PMID: 16368527 Review.
-
Regulation and function of the human HSP90AA1 gene.Gene. 2015 Oct 1;570(1):8-16. doi: 10.1016/j.gene.2015.06.018. Epub 2015 Jun 10. Gene. 2015. PMID: 26071189 Free PMC article. Review.
Cited by
-
Immunophenotype profile by flow cytometry reveals different subtypes of extracellular vesicles in porcine seminal plasma.Cell Commun Signal. 2024 Jan 23;22(1):63. doi: 10.1186/s12964-024-01485-1. Cell Commun Signal. 2024. PMID: 38263049 Free PMC article.
-
Seminal prolactin is associated with HSP90 transcript content in ejaculated spermatozoa.Clin Exp Reprod Med. 2023 Jun;50(2):99-106. doi: 10.5653/cerm.2022.05757. Epub 2023 May 15. Clin Exp Reprod Med. 2023. PMID: 37258103 Free PMC article.
-
Hsp90 modulates human sperm capacitation via the Erk1/2 and p38 MAPK signaling pathways.Reprod Biol Endocrinol. 2021 Mar 4;19(1):39. doi: 10.1186/s12958-021-00723-2. Reprod Biol Endocrinol. 2021. PMID: 33663544 Free PMC article.
-
Plasmodium female gamete surface HSP90 is a key determinant for fertilization.mBio. 2024 Feb 14;15(2):e0314223. doi: 10.1128/mbio.03142-23. Epub 2023 Dec 22. mBio. 2024. PMID: 38131664 Free PMC article.
-
The proteomic landscape of sperm surface deciphers its maturational and functional aspects in buffalo.Front Physiol. 2024 Jun 28;15:1413817. doi: 10.3389/fphys.2024.1413817. eCollection 2024. Front Physiol. 2024. PMID: 39005499 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

