Comparative pathology of rhesus macaque and common marmoset animal models with Middle East respiratory syndrome coronavirus
- PMID: 28234937
- PMCID: PMC5325479
- DOI: 10.1371/journal.pone.0172093
Comparative pathology of rhesus macaque and common marmoset animal models with Middle East respiratory syndrome coronavirus
Abstract
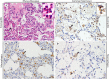
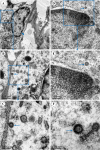
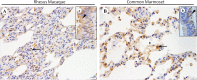
Middle East respiratory syndrome (MERS), which is caused by a newly discovered coronavirus (CoV), has recently emerged. It causes severe viral pneumonia and is associated with a high fatality rate. However, the pathogenesis, comparative pathology and inflammatory cell response of rhesus macaques and common marmosets experimentally infected with MERS-CoV are unknown. We describe the histopathological, immunohistochemical, and ultrastructural findings from rhesus macaque and common marmoset animal models of MERS-CoV infection. The main histopathological findings in the lungs of rhesus macaques and common marmosets were varying degrees of pulmonary lesions, including pneumonia, pulmonary oedema, haemorrhage, degeneration and necrosis of the pneumocytes and bronchial epithelial cells, and inflammatory cell infiltration. The characteristic inflammatory cells in the lungs of rhesus macaques and common marmosets were eosinophils and neutrophils, respectively. Based on these observations, the lungs of rhesus macaques and common marmosets appeared to develop chronic and acute pneumonia, respectively. MERS-CoV antigens and viral RNA were identified in type I and II pneumocytes, alveolar macrophages and bronchial epithelial cells, and ultrastructural observations showed that viral protein was found in type II pneumocytes and inflammatory cells in both species. Correspondingly, the entry receptor DDP4 was found in type I and II pneumocytes, bronchial epithelial cells, and alveolar macrophages. The rhesus macaque and common marmoset animal models of MERS-CoV can be used as a tool to mimic the oncome of MERS-CoV infections in humans. These models can help to provide a better understanding of the pathogenic process of this virus and to develop effective medications and prophylactic treatments.
Conflict of interest statement
Figures


Similar articles
-
An Acute Immune Response to Middle East Respiratory Syndrome Coronavirus Replication Contributes to Viral Pathogenicity.Am J Pathol. 2016 Mar;186(3):630-8. doi: 10.1016/j.ajpath.2015.10.025. Epub 2015 Dec 24. Am J Pathol. 2016. PMID: 26724387 Free PMC article.
-
A Comparative Review of Animal Models of Middle East Respiratory Syndrome Coronavirus Infection.Vet Pathol. 2016 May;53(3):521-31. doi: 10.1177/0300985815620845. Epub 2016 Feb 11. Vet Pathol. 2016. PMID: 26869154 Review.
-
Pathogenicity and Viral Shedding of MERS-CoV in Immunocompromised Rhesus Macaques.Front Immunol. 2018 Feb 12;9:205. doi: 10.3389/fimmu.2018.00205. eCollection 2018. Front Immunol. 2018. PMID: 29483914 Free PMC article.
-
Animal models of Middle East respiratory syndrome coronavirus infection.Antiviral Res. 2015 Oct;122:28-38. doi: 10.1016/j.antiviral.2015.07.005. Epub 2015 Jul 17. Antiviral Res. 2015. PMID: 26192750 Free PMC article. Review.
-
Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease.J Virol. 2015 Apr;89(7):3659-70. doi: 10.1128/JVI.03427-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589660 Free PMC article.
Cited by
-
Middle East Respiratory Syndrome (MERS) Virus-Pathophysiological Axis and the Current Treatment Strategies.AAPS PharmSciTech. 2021 Jun 8;22(5):173. doi: 10.1208/s12249-021-02062-2. AAPS PharmSciTech. 2021. PMID: 34105037 Free PMC article. Review.
-
Immunoediting in SARS-CoV-2: Mutual relationship between the virus and the host.Int Immunopharmacol. 2022 Apr;105:108531. doi: 10.1016/j.intimp.2022.108531. Epub 2022 Jan 10. Int Immunopharmacol. 2022. PMID: 35074569 Free PMC article. Review.
-
Mechanistic Understanding of Lung Inflammation: Recent Advances and Emerging Techniques.J Inflamm Res. 2022 Jun 15;15:3501-3546. doi: 10.2147/JIR.S282695. eCollection 2022. J Inflamm Res. 2022. PMID: 35734098 Free PMC article. Review.
-
Comparison of Experimental Middle East Respiratory Syndrome Coronavirus Infection Acquired by Three Individual Routes of Infection in the Common Marmoset.J Virol. 2022 Feb 23;96(4):e0173921. doi: 10.1128/JVI.01739-21. Epub 2021 Dec 15. J Virol. 2022. PMID: 34908447 Free PMC article.
-
The characteristics of hDPP4 transgenic mice subjected to aerosol MERS coronavirus infection via an animal nose-only exposure device.Animal Model Exp Med. 2019 Oct 30;2(4):269-281. doi: 10.1002/ame2.12088. eCollection 2019 Dec. Animal Model Exp Med. 2019. PMID: 31942559 Free PMC article.
References
-
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, et al. (2013) Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 13: 752–761. 10.1016/S1473-3099(13)70204-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

