A Conjugate Based on Anti-HER2 Diaffibody and Auristatin E Targets HER2-Positive Cancer Cells
- PMID: 28216573
- PMCID: PMC5343935
- DOI: 10.3390/ijms18020401
A Conjugate Based on Anti-HER2 Diaffibody and Auristatin E Targets HER2-Positive Cancer Cells
Erratum in
-
Correction: Serwotka-Suszczak, A. M. et al. A Conjugate Based on Anti-HER2 Diaffibody and Auristatin E Targets HER2-Positive Cancer Cells. Int. J. Mol. Sci. 2017, 18, 401.Int J Mol Sci. 2018 Nov 20;19(11):3676. doi: 10.3390/ijms19113676. Int J Mol Sci. 2018. PMID: 30463379 Free PMC article.
Abstract
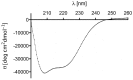
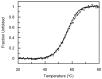
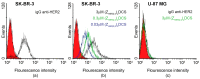
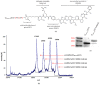
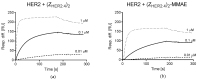
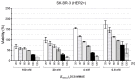
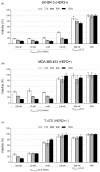
Antibody-drug conjugates (ADCs) have recently emerged as efficient and selective cancer treatment therapeutics. Currently, alternative forms of drug carriers that can replace monoclonal antibodies are under intensive investigation. Here, a cytotoxic conjugate of an anti-HER2 (Human Epidermal Growth Factor Receptor 2) diaffibody with monomethyl-auristatin E (MMAE) is proposed as a potential anticancer therapeutic. The anti-HER2 diaffibody was based on the ZHER2:4 affibody amino acid sequence. The anti-HER2 diaffibody has been expressed as a His-tagged protein in E. coli and purified by Ni-nitrilotriacetyl (Ni-NTA) agarose chromatography. The molecule was properly folded, and the high affinity and specificity of its interaction with HER2 was confirmed by surface plasmon resonance (SPR) and flow cytometry, respectively. The (ZHER2:4)₂DCS-MMAE conjugate was obtained by coupling the maleimide group linked with MMAE to cysteines, which were introduced in a drug conjugation sequence (DCS). Cytotoxicity of the conjugate was evaluated using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide MTT assay and the xCELLigence Real-Time Cell Analyzer. Our experiments demonstrated that the conjugate delivered auristatin E specifically to HER2-positive tumor cells, which finally led to their death. These results indicate that the cytotoxic diaffibody conjugate is a highly potent molecule for the treatment of various types of cancer overexpressing HER2 receptors.
Keywords: HER2; diaffibody; monomethyl auristatin E (MMAE); targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Trastuzumab-monomethyl auristatin E conjugate exhibits potent cytotoxic activity in vitro against HER2-positive human breast cancer.J Cell Physiol. 2019 Mar;234(3):2693-2704. doi: 10.1002/jcp.27085. Epub 2018 Sep 24. J Cell Physiol. 2019. PMID: 30246298
-
Development and biological assessment of MMAE-trastuzumab antibody-drug conjugates (ADCs).Breast Cancer. 2021 Jan;28(1):216-225. doi: 10.1007/s12282-020-01153-5. Epub 2020 Sep 5. Breast Cancer. 2021. PMID: 32889587
-
Antibody-drug conjugates with HER2-targeting antibodies from synthetic antibody libraries are highly potent against HER2-positive human gastric tumor in xenograft models.MAbs. 2019 Jan;11(1):153-165. doi: 10.1080/19420862.2018.1541370. Epub 2018 Nov 8. MAbs. 2019. PMID: 30365359 Free PMC article.
-
Antibody-Drug Conjugate Payloads; Study of Auristatin Derivatives.Chem Pharm Bull (Tokyo). 2020;68(3):201-211. doi: 10.1248/cpb.c19-00853. Chem Pharm Bull (Tokyo). 2020. PMID: 32115527 Review.
-
[Design of next generation antibody drug conjugates].Yao Xue Xue Bao. 2013 Jul;48(7):1053-70. Yao Xue Xue Bao. 2013. PMID: 24133971 Review. Chinese.
Cited by
-
Miniproteins as a Powerful Modality in Drug Development.Trends Biochem Sci. 2020 Apr;45(4):332-346. doi: 10.1016/j.tibs.2019.12.008. Epub 2020 Jan 31. Trends Biochem Sci. 2020. PMID: 32014389 Free PMC article. Review.
-
Site-Specific, Stoichiometric-Controlled, PEGylated Conjugates of Fibroblast Growth Factor 2 (FGF2) with Hydrophilic Auristatin Y for Highly Selective Killing of Cancer Cells Overproducing Fibroblast Growth Factor Receptor 1 (FGFR1).Mol Pharm. 2020 Jul 6;17(7):2734-2748. doi: 10.1021/acs.molpharmaceut.0c00419. Epub 2020 Jun 16. Mol Pharm. 2020. PMID: 32501706 Free PMC article.
-
A novel HER2 targeting nanoagent self-assembled from affibody-epothilone B conjugate for cancer therapy.J Nanobiotechnology. 2024 Aug 21;22(1):502. doi: 10.1186/s12951-024-02754-4. J Nanobiotechnology. 2024. PMID: 39169343 Free PMC article.
-
A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging.Int J Mol Sci. 2017 Jun 7;18(6):1214. doi: 10.3390/ijms18061214. Int J Mol Sci. 2017. PMID: 28590411 Free PMC article.
-
Correction: Serwotka-Suszczak, A. M. et al. A Conjugate Based on Anti-HER2 Diaffibody and Auristatin E Targets HER2-Positive Cancer Cells. Int. J. Mol. Sci. 2017, 18, 401.Int J Mol Sci. 2018 Nov 20;19(11):3676. doi: 10.3390/ijms19113676. Int J Mol Sci. 2018. PMID: 30463379 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

