Paxillin: a crossroad in pathological cell migration
- PMID: 28214467
- PMCID: PMC5316197
- DOI: 10.1186/s13045-017-0418-y
Paxillin: a crossroad in pathological cell migration
Abstract
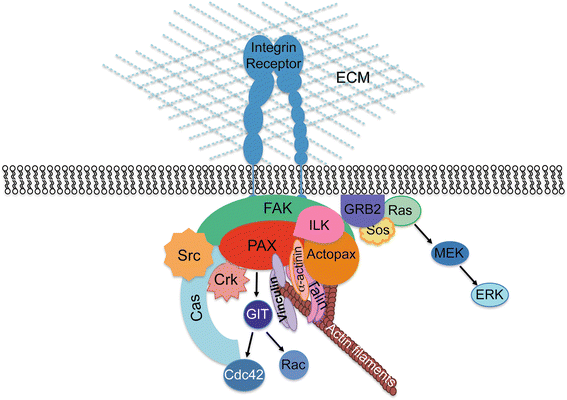
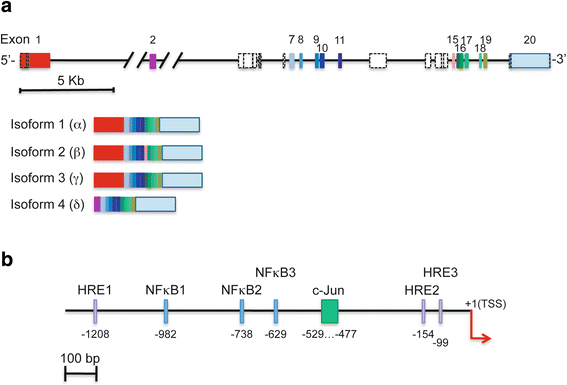
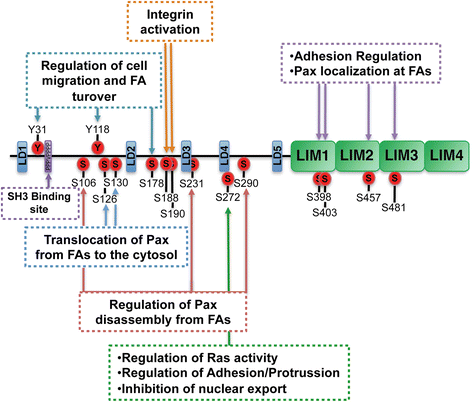
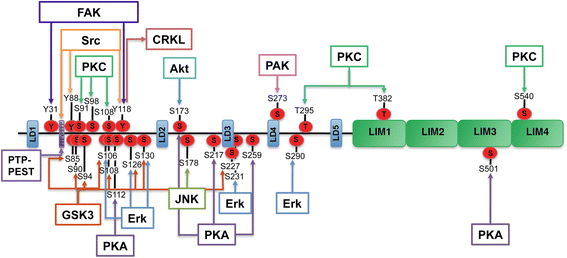
Paxilllin is a multifunctional and multidomain focal adhesion adapter protein which serves an important scaffolding role at focal adhesions by recruiting structural and signaling molecules involved in cell movement and migration, when phosphorylated on specific Tyr and Ser residues. Upon integrin engagement with extracellular matrix, paxillin is phosphorylated at Tyr31, Tyr118, Ser188, and Ser190, activating numerous signaling cascades which promote cell migration, indicating that the regulation of adhesion dynamics is under the control of a complex display of signaling mechanisms. Among them, paxillin disassembly from focal adhesions induced by extracellular regulated kinase (ERK)-mediated phosphorylation of serines 106, 231, and 290 as well as the binding of the phosphatase PEST to paxillin have been shown to play a key role in cell migration. Paxillin also coordinates the spatiotemporal activation of signaling molecules, including Cdc42, Rac1, and RhoA GTPases, by recruiting GEFs, GAPs, and GITs to focal adhesions. As a major participant in the regulation of cell movement, paxillin plays distinct roles in specific tissues and developmental stages and is involved in immune response, epithelial morphogenesis, and embryonic development. Importantly, paxillin is also an essential player in pathological conditions including oxidative stress, inflammation, endothelial cell barrier dysfunction, and cancer development and metastasis.
Keywords: Cancer; Cell migration; Focal adhesions; Signal transduction.
Figures
Similar articles
-
Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration.J Cell Biol. 2002 Nov 25;159(4):673-83. doi: 10.1083/jcb.200202117. J Cell Biol. 2002. PMID: 12446743 Free PMC article.
-
Focal adhesion disassembly is regulated by a RIAM to MEK-1 pathway.J Cell Sci. 2012 Nov 15;125(Pt 22):5338-52. doi: 10.1242/jcs.105270. Epub 2012 Sep 3. J Cell Sci. 2012. PMID: 22946047
-
Paxillin phosphorylation at serine 273 and its effects on Rac, Rho and adhesion dynamics.PLoS Comput Biol. 2018 Jul 5;14(7):e1006303. doi: 10.1371/journal.pcbi.1006303. eCollection 2018 Jul. PLoS Comput Biol. 2018. PMID: 29975690 Free PMC article.
-
The explorations of dynamic interactions of paxillin at the focal adhesions.Biochim Biophys Acta Proteins Proteom. 2022 Oct 1;1870(10):140825. doi: 10.1016/j.bbapap.2022.140825. Epub 2022 Aug 1. Biochim Biophys Acta Proteins Proteom. 2022. PMID: 35926716 Review.
-
Paxillin comes of age.J Cell Sci. 2008 Aug 1;121(Pt 15):2435-44. doi: 10.1242/jcs.018044. J Cell Sci. 2008. PMID: 18650496 Free PMC article. Review.
Cited by
-
Mechanosensing dysregulation in the fibroblast: A hallmark of the aging heart.Ageing Res Rev. 2020 Nov;63:101150. doi: 10.1016/j.arr.2020.101150. Epub 2020 Aug 23. Ageing Res Rev. 2020. PMID: 32846223 Free PMC article. Review.
-
Identification of lymphocyte cell-specific protein-tyrosine kinase (LCK) as a driver for invasion and migration of oral cancer by tumor heterogeneity exploitation.Mol Cancer. 2021 Jun 11;20(1):88. doi: 10.1186/s12943-021-01384-w. Mol Cancer. 2021. PMID: 34116687 Free PMC article.
-
Gene expression profile analysis of the rabbit retinal vein occlusion model.PLoS One. 2020 Jul 31;15(7):e0236928. doi: 10.1371/journal.pone.0236928. eCollection 2020. PLoS One. 2020. PMID: 32735610 Free PMC article.
-
Altered Actin Dynamics in Cell Migration of GNE Mutant Cells.Front Cell Dev Biol. 2021 Mar 18;9:603742. doi: 10.3389/fcell.2021.603742. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816461 Free PMC article.
-
C9orf72-Derived Proline:Arginine Poly-Dipeptides Modulate Cytoskeleton and Mechanical Stress Response.Front Cell Dev Biol. 2022 Mar 23;10:750829. doi: 10.3389/fcell.2022.750829. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35399536 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

