Muscle Yap Is a Regulator of Neuromuscular Junction Formation and Regeneration
- PMID: 28213440
- PMCID: PMC5373129
- DOI: 10.1523/JNEUROSCI.2934-16.2017
Muscle Yap Is a Regulator of Neuromuscular Junction Formation and Regeneration
Erratum in
-
Correction: Zhao et al., "Muscle Yap Is a Regulator of Neuromuscular Junction Formation and Regeneration".J Neurosci. 2017 May 3;37(18):4859. doi: 10.1523/JNEUROSCI.1019-17.2017. J Neurosci. 2017. PMID: 28469011 Free PMC article. No abstract available.
Abstract
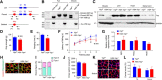
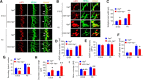
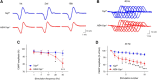
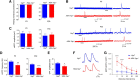
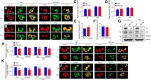
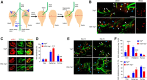
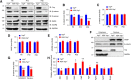
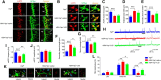
Yes-associated protein (Yap) is a major effector of the Hippo pathway that regulates cell proliferation and differentiation during development and restricts tissue growth in adult animals. However, its role in synapse formation remains poorly understood. In this study, we characterized Yap's role in the formation of the neuromuscular junction (NMJ). In HSA-Yap-/- mice where Yap was mutated specifically in muscle cells, AChR clusters were smaller and were distributed in a broader region in the middle of muscle fibers, suggesting that muscle Yap is necessary for the size and location of AChR clusters. In addition, HSA-Yap-/- mice also exhibited remarkable presynaptic deficits. Many AChR clusters were not or less covered by nerve terminals; miniature endplate potential frequency was reduced, which was associated with an increase in paired-pulse facilitation, indicating structural and functional defects. In addition, muscle Yap mutation prevented reinnervation of denervated muscle fibers. Together, these observations indicate a role of muscle Yap in NMJ formation and regeneration. We found that β-catenin was reduced in the cytoplasm and nucleus of mutant muscles, suggesting compromised β-catenin signaling. Both NMJ formation and regeneration deficits of HSA-Yap-/- mice were ameliorated by inhibiting β-catenin degradation, further corroborating a role of β-catenin or Wnt-dependent signaling downstream of Yap to regulate NMJ formation and regeneration.SIGNIFICANCE STATEMENT This paper explored the role of Yes-associated protein (Yap) in neuromuscular junction (NMJ) formation and regeneration. Yap is a major effector of the Hippo pathway that regulates cell proliferation and differentiation during development and restricts tissue growth in adult animals. However, its role in synapse formation remains poorly understood. We provide evidence that muscle Yap mutation impairs both postsynaptic and presynaptic differentiation and function and inhibits NMJ regeneration after nerve injury, indicating a role of muscle Yap in these events. Further studies suggest compromised β-catenin signaling as a potential mechanism. Both NMJ formation and regeneration deficits of HSA-Yap-/- mice were ameliorated by inhibiting β-catenin degradation, corroborating a role of β-catenin or Wnt-dependent signaling downstream of Yap to regulate NMJ formation and regeneration.
Keywords: YAP; neuromuscular junction; regeneration; β-catenin.
Copyright © 2017 the authors 0270-6474/17/373465-13$15.00/0.
Figures

Similar articles
-
Schwann Cells in Neuromuscular Junction Formation and Maintenance.J Neurosci. 2016 Sep 21;36(38):9770-81. doi: 10.1523/JNEUROSCI.0174-16.2016. J Neurosci. 2016. PMID: 27656017 Free PMC article.
-
β-Catenin gain of function in muscles impairs neuromuscular junction formation.Development. 2012 Jul;139(13):2392-404. doi: 10.1242/dev.080705. Epub 2012 May 23. Development. 2012. PMID: 22627288 Free PMC article.
-
SETD7 Controls Intestinal Regeneration and Tumorigenesis by Regulating Wnt/β-Catenin and Hippo/YAP Signaling.Dev Cell. 2016 Apr 4;37(1):47-57. doi: 10.1016/j.devcel.2016.03.002. Dev Cell. 2016. PMID: 27046831
-
Crosstalk between Agrin and Wnt signaling pathways in development of vertebrate neuromuscular junction.Dev Neurobiol. 2014 Aug;74(8):828-38. doi: 10.1002/dneu.22190. Epub 2014 Jun 4. Dev Neurobiol. 2014. PMID: 24838312 Review.
-
Dual roles for Wnt signalling during the formation of the vertebrate neuromuscular junction.Acta Physiol (Oxf). 2012 Jan;204(1):128-36. doi: 10.1111/j.1748-1716.2011.02295.x. Epub 2011 May 7. Acta Physiol (Oxf). 2012. PMID: 21554559 Review.
Cited by
-
YAP promotes neural crest emigration through interactions with BMP and Wnt activities.Cell Commun Signal. 2019 Jun 22;17(1):69. doi: 10.1186/s12964-019-0383-x. Cell Commun Signal. 2019. PMID: 31228951 Free PMC article.
-
The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease.Int J Mol Sci. 2021 Jul 28;22(15):8058. doi: 10.3390/ijms22158058. Int J Mol Sci. 2021. PMID: 34360831 Free PMC article. Review.
-
H2A.Z is dispensable for both basal and activated transcription in post-mitotic mouse muscles.Nucleic Acids Res. 2020 May 21;48(9):4601-4613. doi: 10.1093/nar/gkaa157. Nucleic Acids Res. 2020. PMID: 32266374 Free PMC article.
-
Counteractive and cooperative actions of muscle β-catenin and CaV1.1 during early neuromuscular synapse formation.iScience. 2022 Mar 4;25(4):104025. doi: 10.1016/j.isci.2022.104025. eCollection 2022 Apr 15. iScience. 2022. PMID: 35340430 Free PMC article.
-
Lamin-Related Congenital Muscular Dystrophy Alters Mechanical Signaling and Skeletal Muscle Growth.Int J Mol Sci. 2020 Dec 30;22(1):306. doi: 10.3390/ijms22010306. Int J Mol Sci. 2020. PMID: 33396724 Free PMC article.
References
-
- Arpke RW, Darabi R, Mader TL, Zhang Y, Toyama A, Lonetree CL, Nash N, Lowe DA, Perlingeiro RC, Kyba M (2013) A new immuno-, dystrophin-deficient model, the NSG-mdx(4Cv) mouse, provides evidence for functional improvement following allogeneic satellite cell transplantation. Stem Cells 31:1611–1620. 10.1002/stem.1402 - DOI - PMC - PubMed
-
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S (2014) YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158:157–170. 10.1016/j.cell.2014.06.013 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
