A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes
- PMID: 28205335
- PMCID: PMC5363357
- DOI: 10.1002/glia.23125
A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes
Abstract
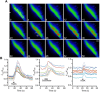
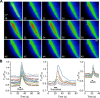
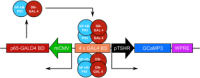
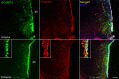
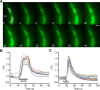
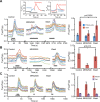
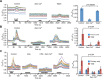
Hypothalamic tanycytes are glial-like glucosensitive cells that contact the cerebrospinal fluid of the third ventricle, and send processes into the hypothalamic nuclei that control food intake and body weight. The mechanism of tanycyte glucosensing remains undetermined. While tanycytes express the components associated with the glucosensing of the pancreatic β cell, they respond to nonmetabolisable glucose analogues via an ATP receptor-dependent mechanism. Here, we show that tanycytes in rodents respond to non-nutritive sweeteners known to be ligands of the sweet taste (Tas1r2/Tas1r3) receptor. The initial sweet tastant-evoked response, which requires the presence of extracellular Ca2+ , leads to release of ATP and a larger propagating Ca2+ response mediated by P2Y1 receptors. In Tas1r2 null mice the proportion of glucose nonresponsive tanycytes was greatly increased in these mice, but a subset of tanycytes retained an undiminished sensitivity to glucose. Our data demonstrate that the sweet taste receptor mediates glucosensing in about 60% of glucosensitive tanycytes while the remaining 40% of glucosensitive tanycytes use some other, as yet unknown mechanism.
Keywords: energy balance; hypothalamus; tanycyte.
© 2017 The Authors GLIA Published by Wiley Periodicals, Inc.
Figures


Similar articles
-
Amino acid sensing in hypothalamic tanycytes via umami taste receptors.Mol Metab. 2017 Nov;6(11):1480-1492. doi: 10.1016/j.molmet.2017.08.015. Epub 2017 Sep 14. Mol Metab. 2017. PMID: 29107294 Free PMC article.
-
ATP-mediated glucosensing by hypothalamic tanycytes.J Physiol. 2011 May 1;589(Pt 9):2275-86. doi: 10.1113/jphysiol.2010.202051. Epub 2011 Mar 8. J Physiol. 2011. PMID: 21486800 Free PMC article.
-
Purinergic signaling in hypothalamic tanycytes: potential roles in chemosensing.Semin Cell Dev Biol. 2011 Apr;22(2):237-44. doi: 10.1016/j.semcdb.2011.02.024. Epub 2011 Mar 21. Semin Cell Dev Biol. 2011. PMID: 21396904 Review.
-
The role of tanycytes in hypothalamic glucosensing.J Cell Mol Med. 2015 Jul;19(7):1471-82. doi: 10.1111/jcmm.12590. Epub 2015 Jun 17. J Cell Mol Med. 2015. PMID: 26081217 Free PMC article. Review.
-
Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing.J Neurochem. 2003 Aug;86(3):709-24. doi: 10.1046/j.1471-4159.2003.01892.x. J Neurochem. 2003. PMID: 12859684
Cited by
-
Glucose transporters in brain in health and disease.Pflugers Arch. 2020 Sep;472(9):1299-1343. doi: 10.1007/s00424-020-02441-x. Epub 2020 Aug 13. Pflugers Arch. 2020. PMID: 32789766 Free PMC article. Review.
-
Are Tanycytes the Missing Link Between Type 2 Diabetes and Alzheimer's Disease?Mol Neurobiol. 2019 Feb;56(2):833-843. doi: 10.1007/s12035-018-1123-8. Epub 2018 May 24. Mol Neurobiol. 2019. PMID: 29797185 Free PMC article. Review.
-
A Short-Term Sucrose Diet Impacts Cell Proliferation of Neural Precursors in the Adult Hypothalamus.Nutrients. 2022 Jun 21;14(13):2564. doi: 10.3390/nu14132564. Nutrients. 2022. PMID: 35807744 Free PMC article.
-
Secretion of Gut Hormones and Expression of Sweet Taste Receptors and Glucose Transporters in a Rat Model of Obesity.Obes Facts. 2019;12(2):190-198. doi: 10.1159/000497122. Epub 2019 Mar 29. Obes Facts. 2019. PMID: 30928977 Free PMC article.
-
[Taste signal transduction and the role of taste receptors in the regulation of microbial infection].Hua Xi Kou Qiang Yi Xue Za Zhi. 2017 Oct 1;35(5):549-554. doi: 10.7518/hxkq.2017.05.020. Hua Xi Kou Qiang Yi Xue Za Zhi. 2017. PMID: 29188655 Free PMC article. Review. Chinese.
References
-
- Curran‐Everett, D. (2000). Multiple comparisons: Philosophies and illustrations. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 279, R1–8. - PubMed
-
- Dale, N. (2011). Purinergic signaling in hypothalamic tanycytes: Potential roles in chemosensing. Seminars in Cell & Developmental Biology, 22, 237–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

