Maternal Binding and Neutralizing IgG Responses Targeting the C-Terminal Region of the V3 Loop Are Predictive of Reduced Peripartum HIV-1 Transmission Risk
- PMID: 28202762
- PMCID: PMC5391478
- DOI: 10.1128/JVI.02422-16
Maternal Binding and Neutralizing IgG Responses Targeting the C-Terminal Region of the V3 Loop Are Predictive of Reduced Peripartum HIV-1 Transmission Risk
Abstract
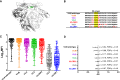
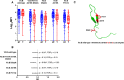
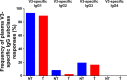
The development of an effective maternal HIV-1 vaccine that could synergize with antiretroviral therapy (ART) to eliminate pediatric HIV-1 infection will require the characterization of maternal immune responses capable of blocking transmission of autologous HIV to the infant. We previously determined that maternal plasma antibody binding to linear epitopes within the variable loop 3 (V3) region of HIV envelope (Env) and neutralizing responses against easy-to-neutralize tier 1 viruses were associated with reduced risk of peripartum HIV infection in the historic U.S. Woman and Infant Transmission Study (WITS) cohort. Here, we defined the fine specificity and function of the potentially protective maternal V3-specific IgG antibodies associated with reduced peripartum HIV transmission risk in this cohort. The V3-specific IgG binding that predicted low risk of mother-to-child-transmission (MTCT) was dependent on the C-terminal flank of the V3 crown and particularly on amino acid position 317, a residue that has also been associated with breakthrough transmission in the RV144 vaccine trial. Remarkably, the fine specificity of potentially protective maternal plasma V3-specific tier 1 virus-neutralizing responses was dependent on the same region in the V3 loop. Our findings suggest that MTCT risk is associated with neutralizing maternal IgG that targets amino acid residues in the C-terminal region of the V3 loop crown, suggesting the importance of the region in immunogen design for maternal vaccines to prevent MTCT.IMPORTANCE Efforts to curb HIV-1 transmission in pediatric populations by antiretroviral therapy (ART) have been highly successful in both developed and developing countries. However, more than 150,000 infants continue to be infected each year, likely due to a combination of late maternal HIV diagnosis, lack of ART access or adherence, and drug-resistant viral strains. Defining the fine specificity of maternal humoral responses that partially protect against MTCT of HIV is required to inform the development of a maternal HIV vaccine that will enhance these responses during pregnancy. In this study, we identified amino acid residues targeted by potentially protective maternal V3-specific IgG binding and neutralizing responses, localizing the potentially protective response in the C-terminal region of the V3 loop crown. Our findings have important implications for the design of maternal vaccination strategies that could synergize with ART during pregnancy to achieve the elimination of pediatric HIV infections.
Keywords: HIV; V3; antibodies; mother-to-child transmission.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Maternal Humoral Immune Correlates of Peripartum Transmission of Clade C HIV-1 in the Setting of Peripartum Antiretrovirals.Clin Vaccine Immunol. 2017 Aug 4;24(8):e00062-17. doi: 10.1128/CVI.00062-17. Print 2017 Aug. Clin Vaccine Immunol. 2017. PMID: 28566336 Free PMC article.
-
Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission.J Clin Invest. 2015 Jul 1;125(7):2702-6. doi: 10.1172/JCI81593. Epub 2015 Jun 8. J Clin Invest. 2015. PMID: 26053661 Free PMC article. Clinical Trial.
-
Maternal Broadly Neutralizing Antibodies Can Select for Neutralization-Resistant, Infant-Transmitted/Founder HIV Variants.mBio. 2020 Mar 10;11(2):e00176-20. doi: 10.1128/mBio.00176-20. mBio. 2020. PMID: 32156815 Free PMC article.
-
Antibodies for prevention of mother-to-child transmission of HIV-1.Curr Opin HIV AIDS. 2015 May;10(3):177-82. doi: 10.1097/COH.0000000000000150. Curr Opin HIV AIDS. 2015. PMID: 25700205 Free PMC article. Review.
-
Passive immunization to prevent mother-infant transmission of human immunodeficiency virus: current issues and future directions.Pediatr Infect Dis J. 1991 Jun;10(6):456-62. doi: 10.1097/00006454-199106000-00009. Pediatr Infect Dis J. 1991. PMID: 1712936 Review.
Cited by
-
Different evolutionary pathways of HIV-1 between fetus and mother perinatal transmission pairs indicate unique immune selection in fetuses.Cell Rep Med. 2021 Jun 16;2(7):100315. doi: 10.1016/j.xcrm.2021.100315. eCollection 2021 Jul 20. Cell Rep Med. 2021. PMID: 34337555 Free PMC article.
-
Neutralization tiers of HIV-1.Curr Opin HIV AIDS. 2018 Mar;13(2):128-136. doi: 10.1097/COH.0000000000000442. Curr Opin HIV AIDS. 2018. PMID: 29266013 Free PMC article. Review.
-
Diversity and Function of Maternal HIV-1-Specific Antibodies at the Time of Vertical Transmission.J Virol. 2020 Apr 16;94(9):e01594-19. doi: 10.1128/JVI.01594-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075936 Free PMC article.
-
Maternal but Not Infant Anti-HIV-1 Neutralizing Antibody Response Associates with Enhanced Transmission and Infant Morbidity.mBio. 2017 Oct 24;8(5):e01373-17. doi: 10.1128/mBio.01373-17. mBio. 2017. PMID: 29066544 Free PMC article.
-
Difficult-to-neutralize global HIV-1 isolates are neutralized by antibodies targeting open envelope conformations.Nat Commun. 2019 Jul 1;10(1):2898. doi: 10.1038/s41467-019-10899-2. Nat Commun. 2019. PMID: 31263112 Free PMC article.
References
-
- UNAIDS. 2015. Progress Report on the Global Plan: towards the elimination of new HIV infections among children and keeping their mothers alive. UNAIDS, Geneva, Switzerland.
-
- Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, Rekacewicz C, Newell ML, Delfraissy JF, Cunningham-Schrader B, Mirochnick M, Sullivan JL. 2002. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA 288:189–198. doi:10.1001/jama.288.2.189. - DOI - PubMed
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL, Jimenez E, O'Neill E, Bazin B, Delfraissy J-F, Culnane M, Coombs R, Elkins M, Moye J, Stratton P, Balsley J, f Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 331:1173–1180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

