Analysis of Chemopredictive Assay for Targeting Cancer Stem Cells in Glioblastoma Patients
- PMID: 28199863
- PMCID: PMC5310181
- DOI: 10.1016/j.tranon.2017.01.008
Analysis of Chemopredictive Assay for Targeting Cancer Stem Cells in Glioblastoma Patients
Abstract
Introduction: The prognosis of glioblastoma (GBM) treated with standard-of-care maximal surgical resection and concurrent adjuvant temozolomide (TMZ)/radiotherapy remains very poor (less than 15 months). GBMs have been found to contain a small population of cancer stem cells (CSCs) that contribute to tumor propagation, maintenance, and treatment resistance. The highly invasive nature of high-grade gliomas and their inherent resistance to therapy lead to very high rates of recurrence. For these reasons, not all patients with similar diagnoses respond to the same chemotherapy, schedule, or dose. Administration of ineffective anticancer therapy is not only costly but more importantly burdens the patient with unnecessary toxicity and selects for the development of resistant cancer cell clones. We have developed a drug response assay (ChemoID) that identifies the most effective chemotherapy against CSCs and bulk of tumor cells from of a panel of potential treatments, offering great promise for individualized cancer management. Providing the treating physician with drug response information on a panel of approved drugs will aid in personalized therapy selections of the most effective chemotherapy for individual patients, thereby improving outcomes. A prospective study was conducted evaluating the use of the ChemoID drug response assay in GBM patients treated with standard of care.
Methods: Forty-one GBM patients (mean age 54 years, 59% male), all eligible for a surgical biopsy, were enrolled in an Institutional Review Board-approved protocol, and fresh tissue samples were collected for drug sensitivity testing. Patients were all treated with standard-of-care TMZ plus radiation with or without maximal surgery, depending on the status of the disease. Patients were prospectively monitored for tumor response, time to recurrence, progression-free survival (PFS), and overall survival (OS). Odds ratio (OR) associations of 12-month recurrence, PFS, and OS outcomes were estimated for CSC, bulk tumor, and combined assay responses for the standard-of-care TMZ treatment; sensitivities/specificities, areas under the curve (AUCs), and risk reclassification components were examined.
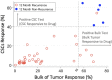
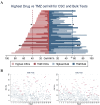
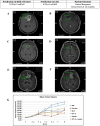
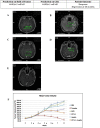
Results: Median follow-up was 8 months (range 3-49 months). For every 5% increase in in vitro CSC cell kill by TMZ, 12-month patient response (nonrecurrence of cancer) increased two-fold, OR=2.2 (P=.016). Similar but somewhat less supported associations with the bulk tumor test were seen, OR=2.75 (P=.07) for each 5% bulk tumor cell kill by TMZ. Combining CSC and bulk tumor assay results in a single model yielded a statistically supported CSC association, OR=2.36 (P=.036), but a much attenuated remaining bulk tumor association, OR=1.46 (P=.472). AUCs and [sensitivity/specificity] at optimal outpoints (>40% CSC cell kill and >55% bulk tumor cell kill) were AUC=0.989 [sensitivity=100/specificity=97], 0.972 [100/89], and 0.989 [100/97] for the CSC only, bulk tumor only, and combined models, respectively. Risk categorization of patients was improved by 11% when using the CSC test in conjunction with the bulk test (risk reclassification nonevent net reclassification improvement [NRI] and overall NRI=0.111, P=.030). Median recurrence time was 20 months for patients with a positive (>40% cell kill) CSC test versus only 3 months for those with a negative CSC test, whereas median recurrence time was 13 months versus 4 months for patients with a positive (>55% cell kill) bulk test versus negative. Similar favorable results for the CSC test were observed for PFS and OS outcomes. Panel results across 14 potential other treatments indicated that 34/41 (83%) potentially more optimal alternative therapies may have been chosen using CSC results, whereas 27/41 (66%) alternative therapies may have been chosen using bulk tumor results.
Conclusions: The ChemoID CSC drug response assay has the potential to increase the accuracy of bulk tumor assays to help guide individualized chemotherapy choices. GBM cancer recurrence may occur quickly if the CSC test has a low in vitro cell kill rate even if the bulk tumor test cell kill rate is high.
Published by Elsevier Inc.
Figures

Similar articles
-
Cancer Stem Cell Chemotherapeutics Assay for Prospective Treatment of Recurrent Glioblastoma and Progressive Anaplastic Glioma: A Single-Institution Case Series.Transl Oncol. 2020 Apr;13(4):100755. doi: 10.1016/j.tranon.2020.100755. Epub 2020 Mar 17. Transl Oncol. 2020. PMID: 32197147 Free PMC article.
-
Treatment of unmethylated MGMT-promoter recurrent glioblastoma with cancer stem cell assay-guided chemotherapy and the impact on patients' healthcare costs.Neurooncol Adv. 2023 May 12;5(1):vdad055. doi: 10.1093/noajnl/vdad055. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37287692 Free PMC article.
-
Cancer Stem Cell Assay for the Treatment of Platinum-Resistant Recurrent Ovarian Cancer.HSOA J Stem Cells Res Dev Ther. 2021;7(3):076. doi: 10.24966/srdt-2060/100076. Epub 2021 Sep 9. HSOA J Stem Cells Res Dev Ther. 2021. PMID: 34796266 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840 Review.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
Cited by
-
Opportunities in Cancer Therapies: Deciphering the Role of Cancer Stem Cells in Tumour Repopulation.Int J Mol Sci. 2023 Dec 8;24(24):17258. doi: 10.3390/ijms242417258. Int J Mol Sci. 2023. PMID: 38139085 Free PMC article. Review.
-
All-trans retinoic acid therapy induces asymmetric division of glioma stem cells from the U87MG cell line.Oncol Lett. 2019 Oct;18(4):3646-3654. doi: 10.3892/ol.2019.10691. Epub 2019 Jul 31. Oncol Lett. 2019. PMID: 31579077 Free PMC article.
-
New Advances and Challenges of Targeting Cancer Stem Cells.Cancer Res. 2017 Oct 1;77(19):5222-5227. doi: 10.1158/0008-5472.CAN-17-0054. Epub 2017 Sep 19. Cancer Res. 2017. PMID: 28928129 Free PMC article.
-
Glioblastoma and the search for non-hypothesis driven combination therapeutics in academia.Front Oncol. 2023 Jan 17;12:1075559. doi: 10.3389/fonc.2022.1075559. eCollection 2022. Front Oncol. 2023. PMID: 36733367 Free PMC article. Review.
-
Cancer stem cell mobilization and therapeutic targeting of the 5T4 oncofetal antigen.Ther Adv Vaccines Immunother. 2019 Jan 25;7:2515135518821623. doi: 10.1177/2515135518821623. eCollection 2019. Ther Adv Vaccines Immunother. 2019. PMID: 30719508 Free PMC article. Review.
References
-
- Delgado-Lopez PD, Corrales-Garcia EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;11:1062–1071. - PubMed
-
- Johnson DR, O'Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. - PubMed
-
- Sundar SJ, Hsieh JK, Manjila S, Lathia JD, Sloan A. The role of cancer stem cells in glioblastoma. Neurosurg Focus. 2014;37(6) - PubMed
-
- Steffens R, Semrau S, Lahmer G, Putz F, Lettmaier S, Eyupoglu I, Buchfelder M, Fietkau R. Recurrent glioblastoma: who receives tumor specific treatment and how often? J Neurooncol. 2016;128(1):85–92. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

