Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells
- PMID: 28199000
- PMCID: PMC5386820
- DOI: 10.1111/epi.13703
Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells
Abstract
Objective: Recent evidence suggests a metabolic contribution of cytochrome P450 enzymes (CYPs) to the drug-resistant phenotype in human epilepsy. However, the upstream molecular regulators of CYP in the epileptic brain remain understudied. We therefore investigated the expression and function of pregnane xenobiotic (PXR) and glucocorticoid (GR) nuclear receptors in endothelial cells established from post-epilepsy surgery brain samples.
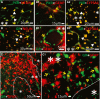
Methods: PXR/GR localization was evaluated by immunohistochemistry in specimens from subjects who underwent temporal lobe resections to relieve drug-resistant seizures. We used primary cultures of endothelial cells obtained from epileptic brain tissues (EPI-ECs; n = 8), commercially available human brain microvascular endothelial cells (HBMECs; n = 8), and human hepatocytes (n = 3). PXR/GR messenger RNA (mRNA) levels in brain ECs was initially determined by complementary DNA (cDNA) microarrays. The expression of PXR/GR proteins was quantified by Western blot. PXR and GR silencing was performed in EPI-ECs (n = 4), and the impact on downstream CYP expression was determined.
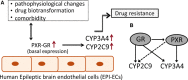
Results: PXR/GR expression was detected by immunofluorescence in ECs and neurons in the human temporal lobe samples analyzed. Elevated mRNA and protein levels of PXR and GR were found in EPI-ECs versus control HBMECs. Hepatocytes, used as a positive control, displayed the highest levels of PXR/GR expression. We confirmed expression of PXR/GR in cytoplasmic-nuclear subcellular fractions, with a significant increase of PXR/GR in EPI-ECs versus controls. CYP3A4, CYP2C9, and CYP2E1 were overexpressed in EPI-ECs versus control, whereas CYP2D6 and CYP2C19 were downregulated or absent in EPI-ECs. GR silencing in EPI-ECs led to decreased CYP3A4, CYP2C9, and PXR expression. PXR silencing in EPI-ECs resulted in the specific downregulation of CYP3A4 expression.
Significance: Our results indicate increased PXR and GR in primary ECs derived from human epileptic brains. PXR or GR may be responsible for a local drug brain metabolism sustained by abnormal CYP regulation.
Keywords: Drug resistance; Epilepsy; Neurovascular unit; Nuclear Receptors.
© 2017 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Figures




Similar articles
-
Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood-brain barrier model.Epilepsia. 2018 Nov;59(11):2049-2060. doi: 10.1111/epi.14567. Epub 2018 Sep 28. Epilepsia. 2018. PMID: 30264400 Free PMC article.
-
Heat Shock Proteins Accelerate the Maturation of Brain Endothelial Cell Glucocorticoid Receptor in Focal Human Drug-Resistant Epilepsy.Mol Neurobiol. 2020 Nov;57(11):4511-4529. doi: 10.1007/s12035-020-02043-9. Epub 2020 Aug 3. Mol Neurobiol. 2020. PMID: 32748370 Free PMC article.
-
Pattern of P450 expression at the human blood-brain barrier: roles of epileptic condition and laminar flow.Epilepsia. 2010 Aug;51(8):1408-17. doi: 10.1111/j.1528-1167.2009.02428.x. Epub 2010 Jan 13. Epilepsia. 2010. PMID: 20074231 Free PMC article.
-
Interplay of pregnane X receptor with other nuclear receptors on gene regulation.Drug Metab Pharmacokinet. 2008;23(1):14-21. doi: 10.2133/dmpk.23.14. Drug Metab Pharmacokinet. 2008. PMID: 18305371 Review.
-
Mathematical Models in the Description of Pregnane X Receptor (PXR)-Regulated Cytochrome P450 Enzyme Induction.Int J Mol Sci. 2018 Jun 15;19(6):1785. doi: 10.3390/ijms19061785. Int J Mol Sci. 2018. PMID: 29914136 Free PMC article. Review.
Cited by
-
RNA-seq analysis of blood of valproic acid-responsive and non-responsive pediatric patients with epilepsy.Exp Ther Med. 2019 Jul;18(1):373-383. doi: 10.3892/etm.2019.7538. Epub 2019 Apr 30. Exp Ther Med. 2019. PMID: 31258675 Free PMC article.
-
A novel biomimetic nanomedicine system with anti-inflammatory and anti-osteoporosis effects improves the therapy efficacy of steroid-resistant nephrotic syndrome.J Nanobiotechnology. 2021 Dec 13;19(1):417. doi: 10.1186/s12951-021-01165-z. J Nanobiotechnology. 2021. PMID: 34903236 Free PMC article.
-
The role of molecular chaperones in the mechanisms of epileptogenesis.Cell Stress Chaperones. 2023 Nov;28(6):599-619. doi: 10.1007/s12192-023-01378-1. Epub 2023 Sep 27. Cell Stress Chaperones. 2023. PMID: 37755620 Free PMC article. Review.
-
Association of ABCB1 Polymorphisms with Efficacy and Adverse Drug Reactions of Valproic Acid in Children with Epilepsy.Pharmaceuticals (Basel). 2023 Oct 30;16(11):1536. doi: 10.3390/ph16111536. Pharmaceuticals (Basel). 2023. PMID: 38004402 Free PMC article.
-
The constitutive androstane receptor and pregnane X receptor in the brain.Br J Pharmacol. 2020 Jun;177(12):2666-2682. doi: 10.1111/bph.15055. Epub 2020 Apr 22. Br J Pharmacol. 2020. PMID: 32201941 Free PMC article. Review.
References
-
- Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 2004;3:950–964. - PubMed
-
- Tirona RG, Kim RB. Nuclear receptors and drug disposition gene regulation. J Pharm Sci 2005;94:1169–1186. - PubMed
-
- Gerbal‐Chaloin S, Pascussi JM, Pichard‐Garcia L, et al. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos 2001;29:242–251. - PubMed
-
- Pascussi JM, Gerbal‐Chaloin S, Drocourt L, et al. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta 2003;1619:243–253. - PubMed
-
- Pascussi JM, Gerbal‐Chaloin S, Duret C, et al. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol 2008;48:1–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

