Defining the contribution of neuroinflammation to Parkinson's disease in humanized immune system mice
- PMID: 28196514
- PMCID: PMC5310074
- DOI: 10.1186/s13024-017-0158-z
Defining the contribution of neuroinflammation to Parkinson's disease in humanized immune system mice
Abstract
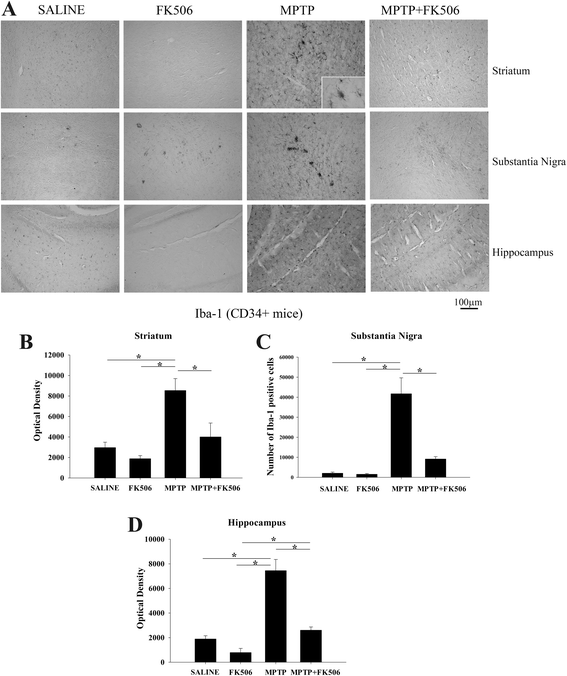
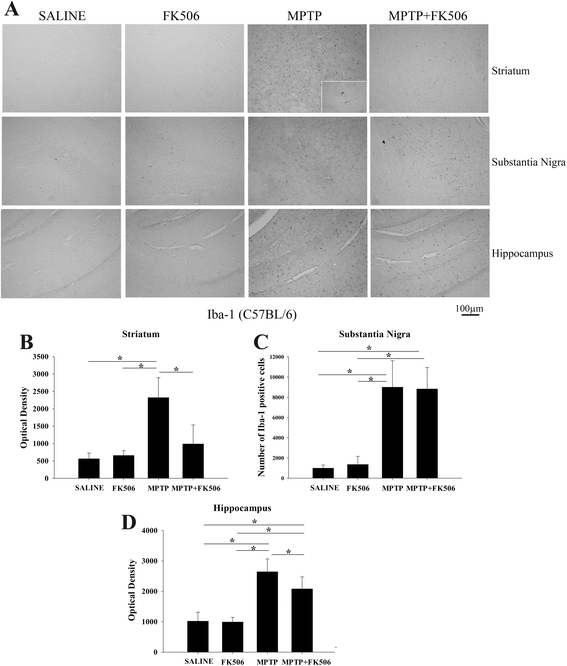
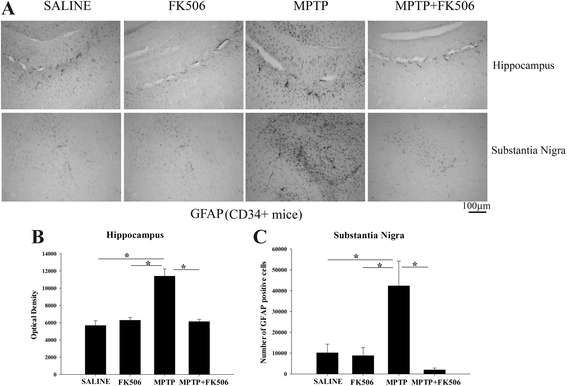
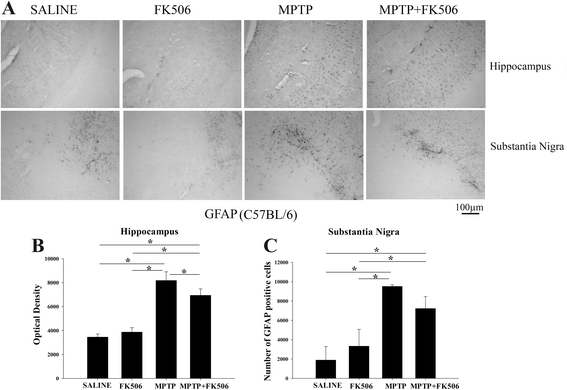
Background: Reactive microglia have been associated with the histological changes that occur in Parkinson's disease brains and mouse models of the disease. Multiple studies from autopsy brains have verified the presence of microgliosis in several brain regions including substantia nigra, striatum, hippocampus and various cortical areas. MPTP injections in rodents have also shown striato-nigral microgliosis correlating with the loss of dopaminergic neurons. However, consistent data with respect to cytokine and immune cell changes during Parkinson's disease have not been fully defined.
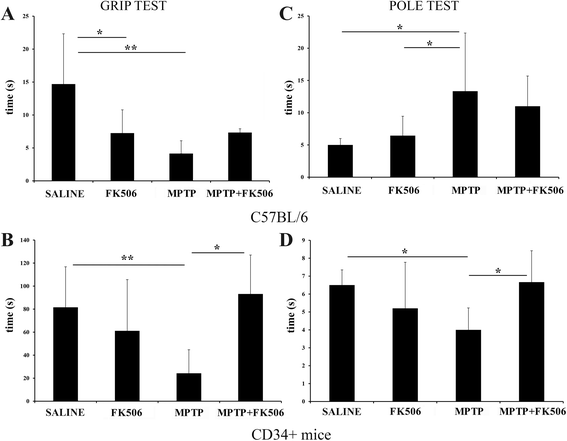
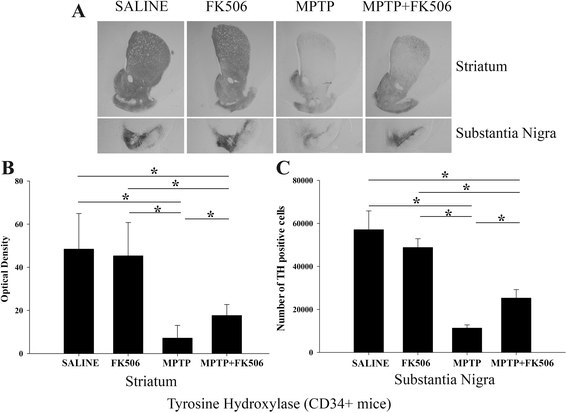
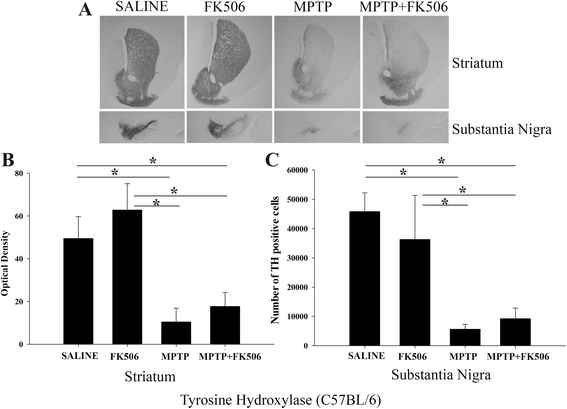
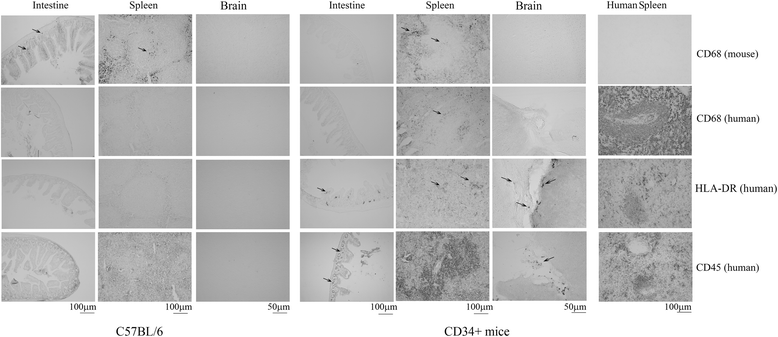
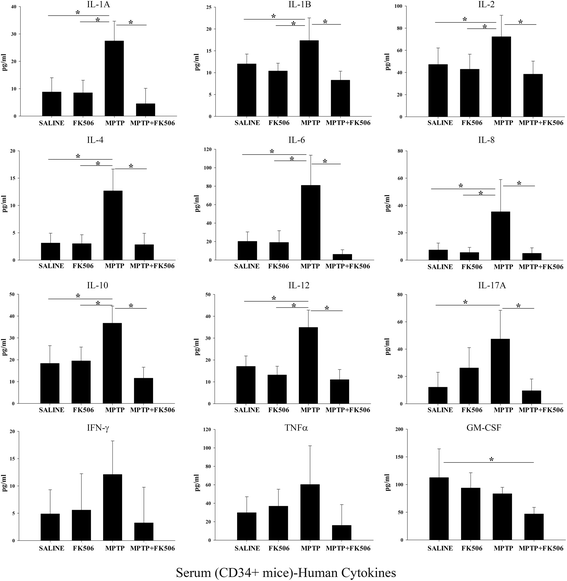
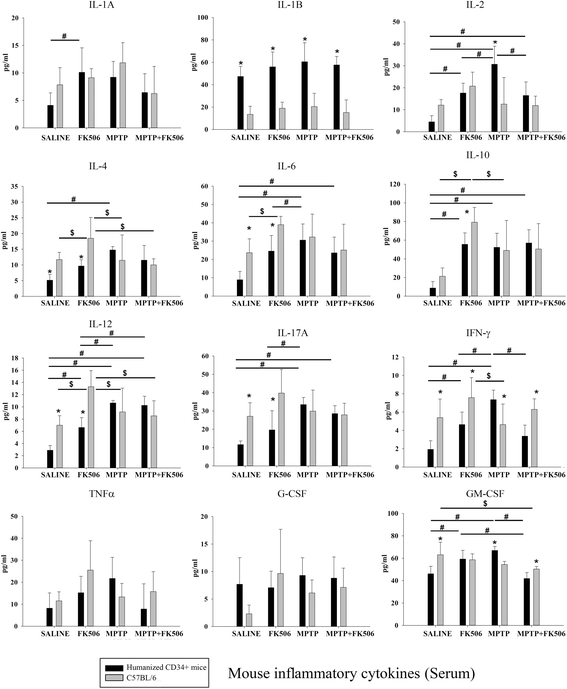
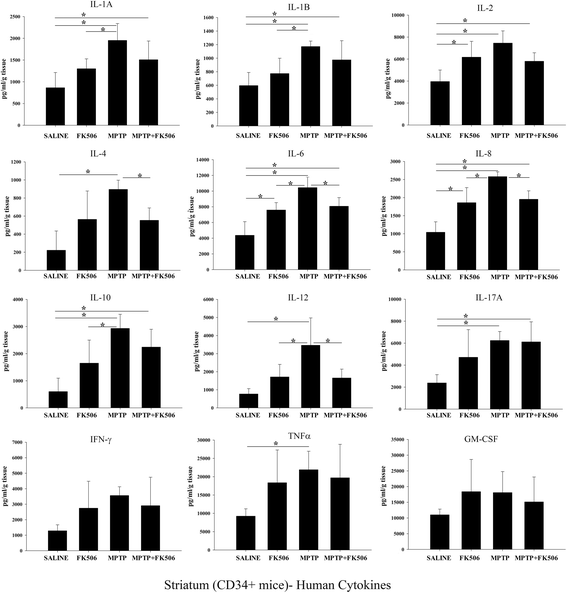
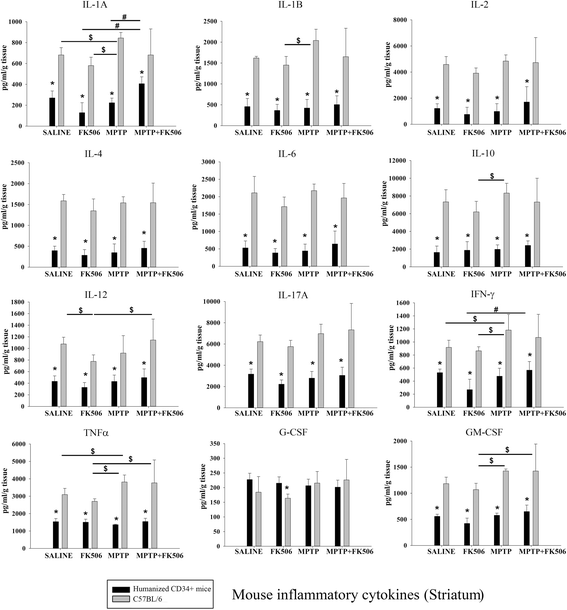
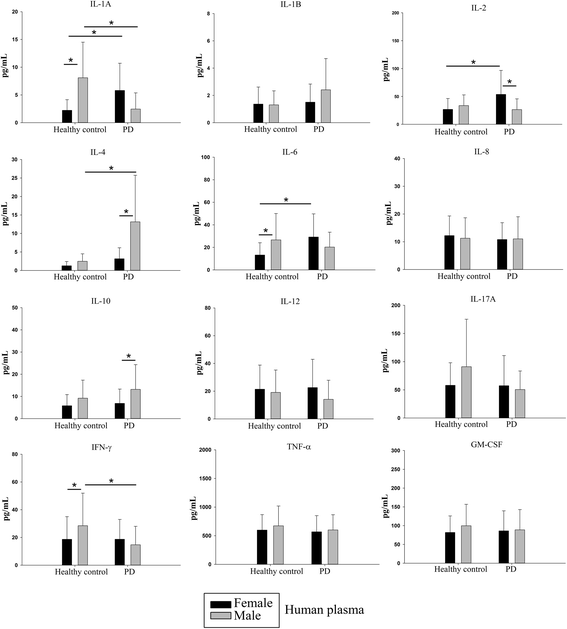
Results: In order to improve understanding of the role of neuroinflammation in Parkinson's disease, we employed the MPTP injection model using humanized CD34+ mice along with age-matched C57BL/6 mice. NSG mice engrafted with hu-CD34+ hematopoietic stem cells were injected with MPTP to quantify cytokine changes, neuron loss, gliosis, and behavioral dysfunction. The mice were also treated with or without the calcineurin/NFAT inhibitor, FK506, to determine whether modulating the immune response could attenuate disease. MPTP injections produced impairment of motor performance, increased microgliosis, elevated brain cytokine levels, and reduced tyrosine hydroxylase immunoreactivity in the substantia nigra and striatum of both humanized CD34+ mice and C57BL/6 mice with a strikingly different profile of human versus mouse cytokine elevations observed in each. Interestingly, FK506 injections significantly attenuated the MPTP-induced effects in the humanized CD34+ mice compared the C57BL/6 mice. In addition, analyses of human plasma from Parkinson's disease donors compared to age-matched, healthy controls demonstrated an increase in a number of pro-inflammatory cytokines in female patients similar to that observed in MPTP-injected female CD34+ mice.
Conclusions: This study demonstrates for the first time, induction of Parkinson's disease-like symptoms in female humanized CD34+ mice using MPTP. The profile of cytokine changes in the serum and brains of the humanized CD34+ mice following MPTP injection differed significantly from that occurring in the more commonly used C57BL/6 strain of mice. Moreover, several cytokine elevations observed in the MPTP injected humanized CD34+ mice were similarly increased in plasma of PD patients suggesting that these mice offer the more relevant model for the inflammatory aspects of human disease. Consistent with this, the effects of MPTP on loss of tyrosine hydroxylase immunoreactivity, loss of motor strength, and increase in proinflammatory cytokines were attenuated using an immunosuppressant drug, FK506, in the humanized CD34+ but not the C57BL/6 mice. Collectively, these findings suggest that MPTP injected, humanized CD34+ mice represent a more accurate model for assessing inflammatory changes in PD.
Keywords: FK506; Humanized mice; MPTP; Neuroinflammation; Parkinson’s disease.
Figures
Similar articles
-
Neuroinflammation in Parkinson's patients and MPTP-treated mice is not restricted to the nigrostriatal system: microgliosis and differential expression of interleukin-1 receptors in the olfactory bulb.Exp Gerontol. 2007 Aug;42(8):762-71. doi: 10.1016/j.exger.2007.04.010. Epub 2007 Apr 29. Exp Gerontol. 2007. PMID: 17592750
-
RANTES-induced invasion of Th17 cells into substantia nigra potentiates dopaminergic cell loss in MPTP mouse model of Parkinson's disease.Neurobiol Dis. 2019 Dec;132:104575. doi: 10.1016/j.nbd.2019.104575. Epub 2019 Aug 22. Neurobiol Dis. 2019. PMID: 31445159 Free PMC article.
-
The α7 nAChR agonist PNU-282987 reduces inflammation and MPTP-induced nigral dopaminergic cell loss in mice.J Parkinsons Dis. 2013;3(2):161-72. doi: 10.3233/JPD-120157. J Parkinsons Dis. 2013. PMID: 23938346
-
Changes in cytokines and neurotrophins in Parkinson's disease.J Neural Transm Suppl. 2000;(60):277-90. doi: 10.1007/978-3-7091-6301-6_19. J Neural Transm Suppl. 2000. PMID: 11205147 Review.
-
Parkinson's disease: Autoimmunity and neuroinflammation.Autoimmun Rev. 2016 Oct;15(10):1005-11. doi: 10.1016/j.autrev.2016.07.022. Epub 2016 Aug 4. Autoimmun Rev. 2016. PMID: 27497913 Review.
Cited by
-
Calmodulin and Its Binding Proteins in Parkinson's Disease.Int J Mol Sci. 2021 Mar 16;22(6):3016. doi: 10.3390/ijms22063016. Int J Mol Sci. 2021. PMID: 33809535 Free PMC article. Review.
-
A Novel Synthetic Derivative of Phloroglucinol Inhibits Neuroinflammatory Responses Through Attenuating Kalirin Signaling Pathway in Murine BV2 Microglial Cells.Mol Neurobiol. 2019 Apr;56(4):2870-2880. doi: 10.1007/s12035-018-1233-3. Epub 2018 Jul 31. Mol Neurobiol. 2019. PMID: 30066307
-
Alpha-Synuclein Induced Immune Cells Activation and Associated Therapy in Parkinson's Disease.Front Aging Neurosci. 2021 Nov 5;13:769506. doi: 10.3389/fnagi.2021.769506. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34803660 Free PMC article. Review.
-
Microglia, inflammation and gut microbiota responses in a progressive monkey model of Parkinson's disease: A case series.Neurobiol Dis. 2020 Oct;144:105027. doi: 10.1016/j.nbd.2020.105027. Epub 2020 Jul 24. Neurobiol Dis. 2020. PMID: 32712266 Free PMC article.
-
Eluted 25-hydroxyvitamin D3 from radially aligned nanofiber scaffolds enhances cathelicidin production while reducing inflammatory response in human immune system-engrafted mice.Acta Biomater. 2019 Oct 1;97:187-199. doi: 10.1016/j.actbio.2019.08.005. Epub 2019 Aug 3. Acta Biomater. 2019. PMID: 31386930 Free PMC article.
References
-
- Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J Neurochem. 2009;110(3):966–975. doi: 10.1111/j.1471-4159.2009.06189.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

