Error-prone meiotic division and subfertility in mice with oocyte-conditional knockdown of pericentrin
- PMID: 28193732
- PMCID: PMC6518315
- DOI: 10.1242/jcs.196188
Error-prone meiotic division and subfertility in mice with oocyte-conditional knockdown of pericentrin
Abstract
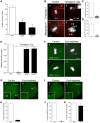
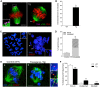
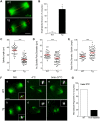
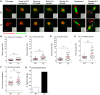
Mouse oocytes lack canonical centrosomes and instead contain unique acentriolar microtubule-organizing centers (aMTOCs). To test the function of these distinct aMTOCs in meiotic spindle formation, pericentrin (Pcnt), an essential centrosome/MTOC protein, was knocked down exclusively in oocytes by using a transgenic RNAi approach. Here, we provide evidence that disruption of aMTOC function in oocytes promotes spindle instability and severe meiotic errors that lead to pronounced female subfertility. Pcnt-depleted oocytes from transgenic (Tg) mice were ovulated at the metaphase-II stage, but show significant chromosome misalignment, aneuploidy and premature sister chromatid separation. These defects were associated with loss of key Pcnt-interacting proteins (γ-tubulin, Nedd1 and Cep215) from meiotic spindle poles, altered spindle structure and chromosome-microtubule attachment errors. Live-cell imaging revealed disruptions in the dynamics of spindle assembly and organization, together with chromosome attachment and congression defects. Notably, spindle formation was dependent on Ran GTPase activity in Pcnt-deficient oocytes. Our findings establish that meiotic division is highly error-prone in the absence of Pcnt and disrupted aMTOCs, similar to what reportedly occurs in human oocytes. Moreover, these data underscore crucial differences between MTOC-dependent and -independent meiotic spindle assembly.
Keywords: MTOC; Meiosis; Microtubule-organizing center; Oocyte; Pericentrin; Spindle.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


Similar articles
-
Loss of acentriolar MTOCs disrupts spindle pole Aurora A and assembly of the liquid-like meiotic spindle domain in oocytes.J Cell Sci. 2021 Jul 15;134(14):jcs256297. doi: 10.1242/jcs.256297. Epub 2021 Jul 20. J Cell Sci. 2021. PMID: 34152366 Free PMC article.
-
CEP215 and AURKA regulate spindle pole focusing and aMTOC organization in mouse oocytes.Reproduction. 2020 Mar;159(3):261-274. doi: 10.1530/REP-19-0263. Reproduction. 2020. PMID: 31895686 Free PMC article.
-
NEDD1 is crucial for meiotic spindle stability and accurate chromosome segregation in mammalian oocytes.Dev Biol. 2010 Mar 15;339(2):439-50. doi: 10.1016/j.ydbio.2010.01.009. Epub 2010 Jan 15. Dev Biol. 2010. PMID: 20079731
-
Meiotic spindle formation in mammalian oocytes: implications for human infertility.Biol Reprod. 2018 Feb 1;98(2):153-161. doi: 10.1093/biolre/iox145. Biol Reprod. 2018. PMID: 29342242 Review.
-
Meeting the meiotic challenge: Specializations in mammalian oocyte spindle formation.Mol Reprod Dev. 2018 Mar;85(3):178-187. doi: 10.1002/mrd.22967. Epub 2018 Mar 5. Mol Reprod Dev. 2018. PMID: 29411912 Free PMC article. Review.
Cited by
-
Participation of EML6 in the regulation of oocyte meiotic progression in mice.J Biomed Res. 2019 Apr 30;34(1):44-53. doi: 10.7555/JBR.33.20190014. J Biomed Res. 2019. PMID: 35081682 Free PMC article.
-
Loss of acentriolar MTOCs disrupts spindle pole Aurora A and assembly of the liquid-like meiotic spindle domain in oocytes.J Cell Sci. 2021 Jul 15;134(14):jcs256297. doi: 10.1242/jcs.256297. Epub 2021 Jul 20. J Cell Sci. 2021. PMID: 34152366 Free PMC article.
-
Aurora kinase A is essential for meiosis in mouse oocytes.PLoS Genet. 2021 Apr 26;17(4):e1009327. doi: 10.1371/journal.pgen.1009327. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33901174 Free PMC article.
-
Spatio-temporal requirements of Aurora kinase A in mouse oocyte meiotic spindle building.iScience. 2024 Jul 3;27(8):110451. doi: 10.1016/j.isci.2024.110451. eCollection 2024 Aug 16. iScience. 2024. PMID: 39081293 Free PMC article.
-
Inhibition of neddylation causes meiotic arrest in mouse oocyte.Cell Cycle. 2019 Jun;18(11):1254-1267. doi: 10.1080/15384101.2019.1617453. Epub 2019 May 21. Cell Cycle. 2019. PMID: 31111756 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

