The Principles of Engineering Immune Cells to Treat Cancer
- PMID: 28187291
- PMCID: PMC5553442
- DOI: 10.1016/j.cell.2017.01.016
The Principles of Engineering Immune Cells to Treat Cancer
Abstract
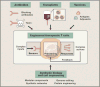
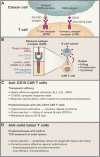
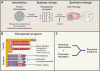
Chimeric antigen receptor (CAR) T cells have proven that engineered immune cells can serve as a powerful new class of cancer therapeutics. Clinical experience has helped to define the major challenges that must be met to make engineered T cells a reliable, safe, and effective platform that can be deployed against a broad range of tumors. The emergence of synthetic biology approaches for cellular engineering is providing us with a broadly expanded set of tools for programming immune cells. We discuss how these tools could be used to design the next generation of smart T cell precision therapeutics.
Copyright © 2017. Published by Elsevier Inc.
Figures


Similar articles
-
T cell engineering as therapy for cancer and HIV: our synthetic future.Philos Trans R Soc Lond B Biol Sci. 2015 Oct 19;370(1680):20140374. doi: 10.1098/rstb.2014.0374. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26416683 Free PMC article. Review.
-
Therapeutic T cell engineering.Nature. 2017 May 24;545(7655):423-431. doi: 10.1038/nature22395. Nature. 2017. PMID: 28541315 Free PMC article. Review.
-
CAR T Cell Therapy for Solid Tumors.Annu Rev Med. 2017 Jan 14;68:139-152. doi: 10.1146/annurev-med-062315-120245. Epub 2016 Nov 17. Annu Rev Med. 2017. PMID: 27860544 Review.
-
Emerging Cellular Therapies for Cancer.Annu Rev Immunol. 2019 Apr 26;37:145-171. doi: 10.1146/annurev-immunol-042718-041407. Epub 2018 Dec 10. Annu Rev Immunol. 2019. PMID: 30526160 Free PMC article. Review.
-
Engineering CAR-T Cells for Next-Generation Cancer Therapy.Cancer Cell. 2020 Oct 12;38(4):473-488. doi: 10.1016/j.ccell.2020.07.005. Epub 2020 Jul 30. Cancer Cell. 2020. PMID: 32735779 Review.
Cited by
-
GUCY2C as a biomarker to target precision therapies for patients with colorectal cancer.Expert Rev Precis Med Drug Dev. 2021;6(2):117-129. doi: 10.1080/23808993.2021.1876518. Epub 2021 Feb 2. Expert Rev Precis Med Drug Dev. 2021. PMID: 34027103 Free PMC article.
-
Next-Generation Manufacturing Protocols Enriching TSCM CAR T Cells Can Overcome Disease-Specific T Cell Defects in Cancer Patients.Front Immunol. 2020 Jun 19;11:1217. doi: 10.3389/fimmu.2020.01217. eCollection 2020. Front Immunol. 2020. PMID: 32636841 Free PMC article.
-
Control of mammalian gene expression by modulation of polyA signal cleavage at 5' UTR.Nat Biotechnol. 2024 Sep;42(9):1454-1466. doi: 10.1038/s41587-023-01989-0. Epub 2024 Jan 2. Nat Biotechnol. 2024. PMID: 38168982
-
Targeting EphA2 in cancer.J Hematol Oncol. 2020 Aug 18;13(1):114. doi: 10.1186/s13045-020-00944-9. J Hematol Oncol. 2020. PMID: 32811512 Free PMC article. Review.
-
Creatine uptake regulates CD8 T cell antitumor immunity.J Exp Med. 2019 Dec 2;216(12):2869-2882. doi: 10.1084/jem.20182044. Epub 2019 Oct 18. J Exp Med. 2019. PMID: 31628186 Free PMC article.
References
-
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. Human Epidermal Growth Factor Receptor 2 (HER2)-Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015;33:1688–1696. - PMC - PubMed
-
- Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

