Chlamydial Lipoproteins Stimulate Toll-Like Receptors 1/2 Mediated Inflammatory Responses through MyD88-Dependent Pathway
- PMID: 28184217
- PMCID: PMC5266682
- DOI: 10.3389/fmicb.2017.00078
Chlamydial Lipoproteins Stimulate Toll-Like Receptors 1/2 Mediated Inflammatory Responses through MyD88-Dependent Pathway
Abstract
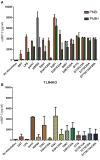
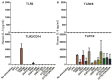
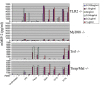
Chlamydiae are very important pathogens which could cause several types of diseases in human, but little is known about its pathogenic mechanism. In order to elucidate host inflammatory response and the signal pathway induced by Chlamydial lipoproteins, the predicted lipoproteins of Chlamydia trachomatis were tested for their ability to induce the release of proinflammatory cytokines by mouse macrophages or human TLR (Toll-Like Receptor) expressing cell lines. The results showed that recombinant proteins of C. trachomatis D381, D541, D067, and D775 displayed a strong ability to induce the release of IL-8 in TLR expressing cell line. The signal pathways involved TLR1/2 and TLR2/CD14 but not TLR4. Moreover, except D067, the proinflammatory cytokine induction by D381, D541, and D775 required the thioacylation site (cysteine) for lipid modification and the induction was through MyD88-mediated pathway. Our data supported that lipoproteins played a vital role in pathogenesis of C. trachomatis-induced inflammatory responses via TLR pathway. It was the first study to characterize other chlamydial lipoproteins after identifying the role of MIP (D541) on pathogenesis of Chlamydial diseases.
Keywords: Chlamydial trachoma; Toll-Like Receptor; cytokine; immune response; lipoproteins.
Figures

Similar articles
-
The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14.J Immunol. 2008 Jan 15;180(2):1158-68. doi: 10.4049/jimmunol.180.2.1158. J Immunol. 2008. PMID: 18178856
-
Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway.J Immunol. 2002 Feb 1;168(3):1435-40. doi: 10.4049/jimmunol.168.3.1435. J Immunol. 2002. PMID: 11801686
-
Expression of TLR 2, TLR 4 and iNOS in cervical monocytes of Chlamydia trachomatis-infected women and their role in host immune response.Am J Reprod Immunol. 2011 Dec;66(6):534-43. doi: 10.1111/j.1600-0897.2011.01064.x. Epub 2011 Aug 24. Am J Reprod Immunol. 2011. PMID: 21883620
-
Role of toll-like receptors in immune responses to chlamydial infections.Curr Pharm Des. 2008;14(6):593-600. doi: 10.2174/138161208783885344. Curr Pharm Des. 2008. PMID: 18336303 Review.
-
Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis.Ann Periodontol. 2002 Dec;7(1):72-8. doi: 10.1902/annals.2002.7.1.72. Ann Periodontol. 2002. PMID: 16013219 Review.
Cited by
-
Conceptual Perspectives: Bacterial Antimicrobial Peptide Induction as a Novel Strategy for Symbiosis with the Human Host.Front Microbiol. 2018 Feb 26;9:302. doi: 10.3389/fmicb.2018.00302. eCollection 2018. Front Microbiol. 2018. PMID: 29535688 Free PMC article.
-
Caveolin-mediated endocytosis of the Chlamydia M278 outer membrane peptide encapsulated in poly(lactic acid)-Poly(ethylene glycol) nanoparticles by mouse primary dendritic cells enhances specific immune effectors mediated by MHC class II and CD4+ T cells.Biomaterials. 2018 Mar;159:130-145. doi: 10.1016/j.biomaterials.2017.12.019. Epub 2017 Dec 26. Biomaterials. 2018. PMID: 29324305 Free PMC article.
-
Treponema denticola dentilisin triggered TLR2/MyD88 activation upregulates a tissue destructive program involving MMPs via Sp1 in human oral cells.PLoS Pathog. 2021 Jul 13;17(7):e1009311. doi: 10.1371/journal.ppat.1009311. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34255809 Free PMC article.
-
Intracellular lifestyle of Chlamydia trachomatis and host-pathogen interactions.Nat Rev Microbiol. 2023 Jul;21(7):448-462. doi: 10.1038/s41579-023-00860-y. Epub 2023 Feb 14. Nat Rev Microbiol. 2023. PMID: 36788308 Review.
-
Recognition of Chlamydia trachomatis by Toll-like receptor 9 is altered during persistence.Infect Immun. 2024 Jul 11;92(7):e0006324. doi: 10.1128/iai.00063-24. Epub 2024 Jun 20. Infect Immun. 2024. PMID: 38899879 Free PMC article.
References
-
- Bas S., Neff L., Vuillet M., Spenato U., Seya T., Matsumoto M., et al. . (2008). The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J. Immunol. 180, 1158–1168. 10.4049/jimmunol.180.2.1158 - DOI - PubMed
-
- Basak C., Pathak S. K., Bhattacharyya A., Pathak S., Basu J., Kundu M. (2005). The secreted peptidyl prolyl cis,trans-isomerase HP0175 of Helicobacter pylori induces apoptosis of gastric epithelial cells in a TLR4- and apoptosis signal-regulating kinase 1-dependent manner. J. Immunol. 174, 5672–5680. 10.4049/jimmunol.174.9.5672 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

