Cardiac diastolic and autonomic dysfunction are aggravated by central chemoreflex activation in heart failure with preserved ejection fraction rats
- PMID: 28181258
- PMCID: PMC5390883
- DOI: 10.1113/JP273558
Cardiac diastolic and autonomic dysfunction are aggravated by central chemoreflex activation in heart failure with preserved ejection fraction rats
Abstract
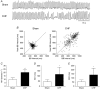
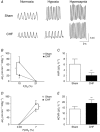
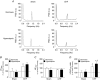
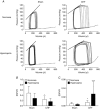
Key points: Heart failure with preserved ejection fraction (HFpEF) is associated with disordered breathing patterns, and sympatho-vagal imbalance. Although it is well accepted that altered peripheral chemoreflex control plays a role in the progression of heart failure with reduced ejection fraction (HFrEF), the pathophysiological mechanisms underlying deterioration of cardiac function in HFpEF are poorly understood. We found that central chemoreflex is enhanced in HFpEF and neuronal activation is increased in pre-sympathetic regions of the brainstem. Our data showed that activation of the central chemoreflex pathway in HFpEF exacerbates diastolic dysfunction, worsens sympatho-vagal imbalance and markedly increases the incidence of cardiac arrhythmias in rats with HFpEF.
Abstract: Heart failure (HF) patients with preserved ejection fraction (HFpEF) display irregular breathing, sympatho-vagal imbalance, arrhythmias and diastolic dysfunction. It has been shown that tonic activation of the central and peripheral chemoreflex pathway plays a pivotal role in the pathophysiology of HF with reduced ejection fraction. In contrast, no studies to date have addressed chemoreflex function or its effect on cardiac function in HFpEF. Therefore, we tested whether peripheral and central chemoreflexes are hyperactive in HFpEF and if chemoreflex activation exacerbates cardiac dysfunction and autonomic imbalance. Sprague-Dawley rats (n = 32) were subjected to sham or volume overload to induce HFpEF. Resting breathing variability, chemoreflex gain, cardiac function and sympatho-vagal balance, and arrhythmia incidence were studied. HFpEF rats displayed [mean ± SD; chronic heart failure (CHF) vs. Sham, respectively] a marked increase in the incidence of apnoeas/hypopnoeas (20.2 ± 4.0 vs. 9.7 ± 2.6 events h-1 ), autonomic imbalance [0.6 ± 0.2 vs. 0.2 ± 0.1 low/high frequency heart rate variability (LF/HFHRV )] and cardiac arrhythmias (196.0 ± 239.9 vs. 19.8 ± 21.7 events h-1 ). Furthermore, HFpEF rats showed increase central chemoreflex sensitivity but not peripheral chemosensitivity. Accordingly, hypercapnic stimulation in HFpEF rats exacerbated increases in sympathetic outflow to the heart (229.6 ± 43.2% vs. 296.0 ± 43.9% LF/HFHRV , normoxia vs. hypercapnia, respectively), incidence of cardiac arrhythmias (196.0 ± 239.9 vs. 576.7 ± 472.9 events h-1 ) and diastolic dysfunction (0.008 ± 0.004 vs. 0.027 ± 0.027 mmHg μl-1 ). Importantly, the cardiovascular consequences of central chemoreflex activation were related to sympathoexcitation since these effects were abolished by propranolol. The present results show that the central chemoreflex is enhanced in HFpEF and that acute activation of central chemoreceptors leads to increases of cardiac sympathetic outflow, cardiac arrhythmogenesis and impairment in cardiac function in rats with HFpEF.
Keywords: autonomic imbalance; cardiac function; central chemoreflex; heart failure preserved ejection fraction; respiratory disorders.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures


Similar articles
-
Exercise training improves cardiac autonomic control, cardiac function, and arrhythmogenesis in rats with preserved-ejection fraction heart failure.J Appl Physiol (1985). 2017 Sep 1;123(3):567-577. doi: 10.1152/japplphysiol.00189.2017. Epub 2017 Jun 15. J Appl Physiol (1985). 2017. PMID: 28620053
-
KLF2 mediates enhanced chemoreflex sensitivity, disordered breathing and autonomic dysregulation in heart failure.J Physiol. 2018 Aug;596(15):3171-3185. doi: 10.1113/JP273805. Epub 2017 Oct 11. J Physiol. 2018. PMID: 29023738 Free PMC article.
-
Rostral ventrolateral medullary catecholaminergic neurones mediate irregular breathing pattern in volume overload heart failure rats.J Physiol. 2019 Dec;597(24):5799-5820. doi: 10.1113/JP278845. Epub 2019 Nov 28. J Physiol. 2019. PMID: 31642520
-
Chemoreflexes--physiology and clinical implications.Acta Physiol Scand. 2003 Mar;177(3):377-84. doi: 10.1046/j.1365-201X.2003.01083.x. Acta Physiol Scand. 2003. PMID: 12609009 Review.
-
Revisiting the physiological effects of exercise training on autonomic regulation and chemoreflex control in heart failure: does ejection fraction matter?Am J Physiol Heart Circ Physiol. 2018 Mar 1;314(3):H464-H474. doi: 10.1152/ajpheart.00407.2017. Epub 2017 Nov 22. Am J Physiol Heart Circ Physiol. 2018. PMID: 29167119 Free PMC article. Review.
Cited by
-
Exercise intolerance in volume overload heart failure is associated with low carotid body mediated chemoreflex drive.Sci Rep. 2021 Jul 14;11(1):14458. doi: 10.1038/s41598-021-93791-8. Sci Rep. 2021. PMID: 34262072 Free PMC article.
-
Medullary astrocytes mediate irregular breathing patterns generation in chronic heart failure through purinergic P2X7 receptor signalling.EBioMedicine. 2022 Jun;80:104044. doi: 10.1016/j.ebiom.2022.104044. Epub 2022 May 9. EBioMedicine. 2022. PMID: 35533501 Free PMC article.
-
Potential Role of the Retrotrapezoid Nucleus in Mediating Cardio-Respiratory Dysfunction in Heart Failure With Preserved Ejection Fraction.Front Physiol. 2022 Apr 12;13:863963. doi: 10.3389/fphys.2022.863963. eCollection 2022. Front Physiol. 2022. PMID: 35492622 Free PMC article. Review.
-
Neurocognitive Disorders in Heart Failure: Novel Pathophysiological Mechanisms Underpinning Memory Loss and Learning Impairment.Mol Neurobiol. 2019 Dec;56(12):8035-8051. doi: 10.1007/s12035-019-01655-0. Epub 2019 Jun 5. Mol Neurobiol. 2019. PMID: 31165973 Review.
-
Contribution of Autonomic Reflexes to the Hyperadrenergic State in Heart Failure.Front Neurosci. 2017 Mar 30;11:162. doi: 10.3389/fnins.2017.00162. eCollection 2017. Front Neurosci. 2017. PMID: 28424575 Free PMC article. Review.
References
-
- Banes AK, Shaw SM, Tawfik A, Patel BP, Ogbi S, Fulton D & Marrero MB (2005). Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin. Am J Physiol Cell Physiol 288, C805–812. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

