Lacrimal Gland Repair Using Progenitor Cells
- PMID: 28170196
- PMCID: PMC5442743
- DOI: 10.5966/sctm.2016-0191
Lacrimal Gland Repair Using Progenitor Cells
Abstract
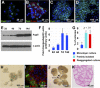
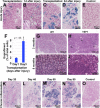
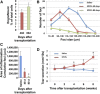
In humans, the lacrimal gland (LG) is the primary contributor to the aqueous layer of the tear film. Production of tears in insufficient quantity or of inadequate quality may lead to aqueous-deficiency dry eye (ADDE). Currently there is no cure for ADDE. The development of strategies to reliably isolate LG stem/progenitor cells from the LG tissue brings great promise for the design of cell replacement therapies for patients with ADDE. We analyzed the therapeutic potential of epithelial progenitor cells (EPCPs) isolated from adult wild-type mouse LGs by transplanting them into the LGs of TSP -1-/- mice, which represent a novel mouse model for ADDE. TSP-1-/- mice are normal at birth but progressively develop a chronic form of ocular surface disease, characterized by deterioration, inflammation, and secretory dysfunction of the lacrimal gland. Our study shows that, among c-kit-positive epithelial cell adhesion molecule (EpCAM+ ) populations sorted from mouse LGs, the c-kit+ dim/EpCAM+ /Sca1 - /CD34 - /CD45 - cells have the hallmarks of an epithelial cell progenitor population. Isolated EPCPs express pluripotency factors and markers of the epithelial cell lineage Runx1 and EpCAM, and they form acini and ducts when grown in reaggregated three-dimensional cultures. Moreover, when transplanted into injured or "diseased" LGs, they engraft into acinar and ductal compartments. EPCP-injected TSP-1-/- LGs showed reduction of cell infiltration, differentiation of the donor EPCPs within secretory acini, and substantial improvement in LG structural integrity and function. This study provides the first evidence for the effective use of adult EPCP cell transplantation to rescue LG dysfunction in a model system. Stem Cells Translational Medicine 2017;6:88-98.
Keywords: Cell culture; Cell surface markers; Cell transplantation; Cellular therapy; Fluorescence-activated cell sorting; Gene expression; Tissue-specific stem cells; c-kit.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

Similar articles
-
Establishment of long-term serum-free culture for lacrimal gland stem cells aiming at lacrimal gland repair.Stem Cell Res Ther. 2020 Jan 8;11(1):20. doi: 10.1186/s13287-019-1541-1. Stem Cell Res Ther. 2020. PMID: 31915062 Free PMC article.
-
Manipulation of Panx1 Activity Increases the Engraftment of Transplanted Lacrimal Gland Epithelial Progenitor Cells.Invest Ophthalmol Vis Sci. 2017 Nov 1;58(13):5654-5665. doi: 10.1167/iovs.17-22071. Invest Ophthalmol Vis Sci. 2017. PMID: 29098296 Free PMC article.
-
Establishing and characterizing lacrispheres from human lacrimal gland for potential clinical application.Graefes Arch Clin Exp Ophthalmol. 2018 Apr;256(4):717-727. doi: 10.1007/s00417-018-3926-8. Epub 2018 Feb 17. Graefes Arch Clin Exp Ophthalmol. 2018. PMID: 29455251
-
The lacrimal gland: development, wound repair and regeneration.Biotechnol Lett. 2017 Jul;39(7):939-949. doi: 10.1007/s10529-017-2326-1. Epub 2017 Mar 28. Biotechnol Lett. 2017. PMID: 28353146 Review.
-
Advances in Functional Restoration of the Lacrimal Glands.Invest Ophthalmol Vis Sci. 2018 Nov 1;59(14):DES174-DES182. doi: 10.1167/iovs.17-23528. Invest Ophthalmol Vis Sci. 2018. PMID: 30481824 Review.
Cited by
-
Origin and Lineage Plasticity of Endogenous Lacrimal Gland Epithelial Stem/Progenitor Cells.iScience. 2020 Jun 26;23(6):101230. doi: 10.1016/j.isci.2020.101230. Epub 2020 Jun 2. iScience. 2020. PMID: 32559730 Free PMC article.
-
The First Transcriptomic Atlas of the Adult Lacrimal Gland Reveals Epithelial Complexity and Identifies Novel Progenitor Cells in Mice.Cells. 2023 May 21;12(10):1435. doi: 10.3390/cells12101435. Cells. 2023. PMID: 37408269 Free PMC article.
-
Lacrimal gland development: From signaling interactions to regenerative medicine.Dev Dyn. 2017 Dec;246(12):970-980. doi: 10.1002/dvdy.24551. Epub 2017 Aug 18. Dev Dyn. 2017. PMID: 28710815 Free PMC article. Review.
-
Establishment of long-term serum-free culture for lacrimal gland stem cells aiming at lacrimal gland repair.Stem Cell Res Ther. 2020 Jan 8;11(1):20. doi: 10.1186/s13287-019-1541-1. Stem Cell Res Ther. 2020. PMID: 31915062 Free PMC article.
-
A Ligation of the Lacrimal Excretory Duct in Mouse Induces Lacrimal Gland Inflammation with Proliferative Cells.Stem Cells Int. 2017;2017:4923426. doi: 10.1155/2017/4923426. Epub 2017 Aug 10. Stem Cells Int. 2017. PMID: 28874911 Free PMC article.
References
-
- Kourembanas S. Stem cell‐based therapy for newborn lung and brain injury: Feasible, safe, and the next therapeutic breakthrough?. J Pediatr 2014;164:954–956. - PubMed
-
- Xu J, Qu J, Cao L et al. Mesenchymal stem cell‐based angiopoietin‐1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol 2008;214:472–481. - PubMed
-
- Buzhor E, Leshansky L, Blumenthal J et al. Cell‐based therapy approaches: The hope for incurable diseases. Regen Med 2014;9:649–672. - PubMed
-
- Burgess KL, Dardick I, Cummins MM et al. Myoepithelial cells actively proliferate during atrophy of rat parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:674–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

